s-BLOCK ELEMENTS - ALKALINE EARTH METALS
ELEMENTS OF GROUP 2
Be - Beryllium Mg - Magnesium Ca - Calcium
Sr - Strontium Ba - Barium Ra - Radium (Radioactive)
These metals are known as alkaline earth metals as their oxides are alkaline and occur in earth crust.
Radium was discovered from the ore pitchblende by Madam Curie. It is used in the treatment of cancer. These metals do not occur in the native form (i.e., do not occur in free state).
GENERAL CHARACTERISTICS
Physical properties of alkaline earth metals are:-
ELECTRONIC CONFIGURATION
Like alkali metals, these are s-block elements, and have two electrons in the valence shell in s-orbital. Hence their electronic configuration may be represented as [noble gas] ns2 where ‘n’ represents the valence shell.
Element
|
Atomic No.
|
Electronic Configuration
|
Valence Shell configuration
|
Be
|
4
|
[He] 2s2
|
2s2
|
Mg
|
12
|
[Ne] 3s2
|
3s2
|
Ca
|
20
|
[Ar] 4s2
|
4s2
|
Sr
|
38
|
[Kr] 5s2
|
5s2
|
Ba
|
56
|
[Xe] 6s2
|
6s2
|
Ra
|
88
|
[Rn] 7s2
|
7s2
|
SIZE OF ATOMS AND IONS (ATOMIC RADII AND IONIC RADII)
- The atomic radii of these elements are quite large but smaller than those of the corresponding elements of group 1, due to increased nuclear charge of these elements which tends to draw the orbital electrons inwards.
- The ionic radii are also large but smaller than those of the alkali metals.
- The atomic as well as ionic radii go on increasing down the group due to the gradual addition of extra energy level and also because of the screening effect.
DENSITY
- These are much denser than alkali metals because of their smaller size and greater nuclear charge.
- The density, however, first decreases from Be to Ca and then steadily increases from Ca to Ra due to difference in crystal structure
MELTING AND BOILING POINTS
- These have higher melting and boiling points than those of alkali metals because the number of bonding electrons in alkaline earth metals is two.
- The melting and boiling points decrease down the group with the exception of magnesium.
- Melting points of halides decrease as the size of the halogen increases. The correct order is
MF2 > MCl2 > MBr2 > MI2
METALLIC PROPERTIES:
They are silvery white metals, soft in nature but harder than alkali metals due to stronger metallic bonding.
ATOMIC VOLUME
Atomic volume of these metals increases considerably on moving from Be to Ra as the atomic radius increases.
IONIZATION ENERGY
- The first I.E. of alkaline earth metals are higher than those of the corresponding alkali metals due to smaller size and higher nuclear charge.
- The second I.E. values are higher than their first I.E. values but much lower than the second I.E. values of alkali metals.
- On moving down the group due to increase in atomic size the magnitude of I.E. decreases.
- The ionization potential of radium is higher than that of barium.
ELECTROPOSITIVE CHARACTER
- These are strong electropositive elements due to their large size and comparatively low ionisation energies.
- On moving down the group, the electropositive character increases due to increase in atomic radii.
OXIDATION STATE
- Alkaline earth metals uniformly show an oxidation state of +2 despite the presence of high ionisation energy because
- In the solid state, the dipositive ions M2+ form strong lattices due to their small size and high charge (i.e., high lattice energy).
- In the aqueous solution, the M2+ cations are strongly hydrated due to their small size and high charge. The hydration energy released by the M2+ cation is very large.
- The divalent ions are diamagnetic and colourless due to the absence of unpaired electron.
CONDUCTIVITY
These are good conductors of heat and electricity due to the presence of two loosely held valence electrons.
FLAME COLOURATION
- Like alkali metal salts, alkaline earth metal salts also impart characteristic flame colouration.
- As we move down the group from Ca to Ba, the ionisation energy decreases, hence the energy or the frequency of the emitted light increases. Thus,
Ca Sr Ba Ra
Brick red Crimson red Apple green Crimson
- Be and Mg because of their high ionization energies, however, do not impart any characteristic colour to the bunsen flame.
CHEMICAL PROPERTIES
- Alkaline earth elements are quite reactive due to their low ionisation energies but are found to be less reactive than alkali metals because the alkaline earth metals have comparatively higher ionisation energy.
- Reactivity of the group 2 elements increases on moving down the group because their ionisation energy decreases.
REACTION WITH WATER
- Group 2 elements are less reactive with water as compared to alkali metals. They react with H2O evolving H2 gas.
- The chemical reactivity of the metal with H2O, however increases as we move from Mg to Ba, i.e., Be does not react even with boiling water and Ba react vigorously even with cold water. Thus, increasing order of reactivity with water is
Mg < Ca < Sr < Ba
REACTION WITH OXYGEN
The affinity for oxygen increases down the group. Thus, Be, Mg and Ca when heated with O2 form monoxides while Sr, Ba and Ra form peroxides.
REACTION WITH ACIDS
- Alkaline earth metals except Be, displace H2 from acids.
- Reactivity, however, increases down the group from Mg to Ba i.e.,Mg < Ca < Sr < Ba
- Only Mg displaces H2 from a very dilute HNO3.
REACTION WITH HYDROGEN
- Except Be, all other elements combine with hydrogen on heating to form hydride (MH2).
- The hydride of beryllium can be prepared indirectly by reducing beryllium chloride with lithium aluminium hydride.
- BeH2 and MgH2 are covalent and polymeric whereas the hydrides of Ca, Sr and Ba are ionic and monomeric in nature.
- CaH2 is also called hydrolith.
- All the hydrides react with water to evolve H2 and thus behave as strong reducing agents.
REACTION WITH HALOGENS
- All the elements of group 2 combine with halogens at high temperature and form their halides (MX2).
- Beryllium halides (BeF2, BeCl2 etc.) are covalent, hygroscopic and fume in air due to hydrolysis. The halides of other alkaline earth metals are fairly ionic and this character increases as the size of the metal increases.
- The halides are soluble in water and their solubility decreases in the order:
MgX2 > CaX2 > SrX2 > BaX2
- BeF2 is very soluble in water due to the high solvation energy of Be2+ in forming
but the fluorides of other alkaline earth metals have high melting point and they are insoluble in water.
- BeCl2 has a polymeric structure in the solid state but exists as a dimer in the vapour state and as a monomer at 1200 K.
REACTION WITH NITROGEN
These metals burn in nitrogen to form nitrides of the types M3N2 which are hydrolysed with water to evolve NH3.
- The ease of formation of nitrides increases from Be to Ba. (Be3N2) is volatile in nature.
- Anhydrous CaCl2 is a good drying agent due to hygroscopic nature (CaCl2.2H2O) and cannot be used to dry alcohol or ammonia as it forms addition products with them.
REACTION WITH CARBON
- When heated with carbon, these form their respective carbides of the general formula MC2 (except Be) and are called acetylides containing the discrete anion.
- Under these conditions beryllium, however, forms Be2C called methanide containing the discrete C4– anion.
- All these carbides are ionic in nature and react with H2O to form acetylene (except Be2C which gives methane).
or 
- On heating MgC2 gives Mg2C3 called allylide which contains the discrete
anion and gives allylene (methyl acetylene) on hydrolysis.
REDUCING CHARACTER
- All the alkaline earth metals, because of their low electrode potentials, are strong reducing agents but these are weaker than the corresponding alkali metals.
- As we move down the group from Be to Ra, the reducing character increases due to decreasing I.E. from Be to Ra.
SOLUBILITY IN LIQUID AMMONIA
- Like alkali metals, these dissolve in liquid ammonia giving coloured solutions.
- The tendency to form ammoniates decreases with increase in size of the metal atom (i.e., on moving down the group).
COMPLEX FORMATION
- Complex formation is favoured in case of alkaline earth metals because of their small sizes as compared to the alkali metals.
- Both Mg2+ and Ca2+ form six coordinate complexes with EDTA (ethylenedi-aminetetracetic acid) which are used to determine the hardness of water.
- Beryllium due to small size forms complexes of type [BeF3]–, [BeF4]–2 [Be (H2O)4]2+.
- Mg exists as a natural complex, chlorophyll where it is complexed with pyrole rings of porphyrin.
BASIC STRENGTH OF OXIDES AND HYDROXIDES
- BeO and Be(OH)2 are amphoteric while the oxides and hydroxides of other alkaline earth metals are basic. The basic strength, however, increases from Be to Ba as the ionisation energy of metal decreases down the group thus the order:
BeO < MgO < CaO < SrO < BaO and
Be(OH)2 < Mg(OH)2 < Ca(OH)2 < Sr(OH)2 < Ba(OH)2
- The basic character of hydroxides of group-2 elements is lesser than those of group-1 hydroxides because of the larger size elements of latter than former group.
- Aq. Ba(OH)2 is known as baryta water.
THERMAL STABILITIES AND NATURE OF BICARBONATES AND CARBONATES
- Bicarbonates of these metals do not exist in solid state but are known in solution only, when these solutions are heated, these get decomposed to evolve CO2.
- The carbonates of alkaline earth metals can be regarded as salts of weak carbonic acid (H2CO3) and metal hydroxide, M(OH)2. The carbonates decompose on heating form metal oxide and CO2.
- The stability of carbonates and bicarbonates increases down the group.
- Carbonates and sulphates of Ca and Mg are responsible for permanent hardness of water while their bicarbonates cause temporary hardness.
SOLUBILITY OF THE SALTS
The solubility of a salt in water depends upon two factors.
- Lattice energy: Higher the magnitude of lattice energy, lesser will be the solubility of the salt in the given solvent.
- Hydration energy: Higher the magnitude of hydration energy, higher will be the solubility of the salt in water (solvent).
- Solubility of hydroxides: As the ionic size of group 2 metals increases from Be to Ba, the lattice energy decreases from Be to Ba, as follows:
- Solubility of sulphates: The solubility of sulphates of alkaline earth metals decreases as we move down the group from Be to Ba due to the reason that ionic size increases down the group. The lattice energy remains constant because sulphate ion is so large, so that small change in cationic sizes do not make any difference. Thus the order:
The negligible solubility of BaSO4 in water is used in both qualitative and quantitative analysis.
- Solubility of carbonates: The solubility of the carbonates in water decreases down the group due to the decrease in the magnitude of hydration energy. However these insoluble metal carbonates are dissolved in water having CO2 as shown:
Mg (HCO3)2  Mg2+ (aq) + 2HCO3– (aq.)
Mg2+ (aq) + 2HCO3– (aq.)
The order of solubility of carbonates:
BeCO3 > MgCO3 > CaCO3 > SrCO3 > BaCO3
ANOMALOUS BEHAVIOUR OF BERYLLIUM
Beryllium, the first member of group 2 differs from the rest of the members of its group due to the following reasons:
- It has a small atomic size as well as small ionic size.
- It has no vacant d-orbitals in valence shell.
- It has high electronegativity value.
- It has a high charge density.
- Its hydration energy is high.
Some points of difference are:
- Be is harder and denser than other members of the group.
- The m.p., b.p., and ionisation energy of Be are the highest of all the alkaline earth metals.
- Be does not react with water even at higher temperature where as other metals do.
- BeO and Be(OH)2 are amphoteric in character whereas oxides and hydroxides of the group 2 metals are basic.
- Be is least metallic of all the alkaline earth metals and forms covalent compounds.
- Be forms nitride Be3N2 with nitrogen which is volatile while nitrides of others are non-volatile.
- Be does not liberate H2 from acids (HCl, H2SO4) where as other metals do.
- Be forms Be2C with carbon while the other members of the group form ionic carbide MC2.
DIAGONAL RELATIONSHIP OR RESEMBLANCE BETWEEN Be AND Al
The first member of group-2, Beryllium, shows similarities in the properties with its diagonally opposite member aluminium of the next group 13 of the next higher period, due to the similar polarizing power. i.e., ionic charge/(ionic radius)2 of Be and Al.
- Both the metals are stable in air.
- Both have a strong tendency to form covalent compounds.
- Both form fluoro complex anions BeF42– and AlF63– in solution.
- With conc. HNO3, both are rendered passive due to the formation of a thin film of their respective oxides on the metal surface.
- Both react with conc. NaOH liberating H2.
- Oxides and hydroxides of both are amphoteric in nature.
- Carbides of both liberate methane on hydrolysis.
- Anhydrous chlorides of both i.e., BeCl2 and AlCl3 act as Lewis acids and dissolve in organic solvents.
- Both do not impart any colour to the flame.
Magnesium along with KClO3 or BaO2 is used in photography flash bulbs, fireworks and as a deoxidiser in metallurgical process.
MgCl2.5MgO xH2O is called Sorel’s cement or Magnesia cement and used to fill the cavities of teeth.
Mg(OH)2 in water is used in medicine as an antacid under the name ‘Milk of Magnesia’, while 12 gm of MgCO3 per 100 c.c. of H2O containing CO2 is known as ‘Fluid Magnesia’.
The finely divided BaSO4 is called Blanc fire and used in paints.
Suspension of slaked lime in water is called white wash (milk of lime).
A solution of MgCl2 + NH4Cl in ammonia is known as Magnesia Mixture.
Plaster of paris CaSO4.1/2 H2O is used in surgery for setting broken bones.
Pure Ca(H2PO4)2 is used as American baking powder.
Gypsum gives different products on heating as
Most abundant alkaline earth metal in the earth’s crust is Ca.
Be and Mg crystallize in hcp, Ca and Sr in ccp and Ba in bcc structures.
Because of comparatively higher electronegativity both Be and Mg form a large number of organometallic compounds.
CaCl2.6H2O is widely used for melting ice on roads, particularly in very cold countries, because a 30% eutectic mixture of CaCl2/H2O freezes at –55ºC as compared with NaCl/H2O at –18ºC.
Magnesium perchlorate, Mg(ClO4)2 is known as anhydrone and used as drying agent.
Mostly kidney stones containing calcium oxalate, CaC2O4.H2O which dissolves in dil. strong acids but remains insoluble in bases.
METALLURGY OF MAGNESIUM
OCCURRENCE AND IMPORTANT MINERALS
- Magnesium occurs in the combined state in nature and it is the essential constituent of chlorophyll, the green colouring matter of the plants.
- The important minerals of magnesium are.
- Magnesite, MgCO3
- Dolomite, MgCO3.CaCO3
- Carnallite, KCl.MgCl2.6H2O
- Epsom salt, MgSO4.7H2O
- Asbestos, CaMg3 (SiO3)4
- Talc, Mg2(Si2O5)2.Mg(OH)2
EXTRACTION
It is extracted by the electrolysis of fused mixture of magnesium chloride (which is obtained from carnallite and magnesite), NaCl and CaCl2 (added to provide conductivity to the electrolyte and to lower the fusion temperature of anhydrous MgCl2) at 700ºC in Dow’s process. In Dow’s process, MgCl2 is obtained from sea water as MgCl2.6H2O which can be changed to anhydrous MgCl2 only by passing dry HCl gas through it because even by strong heating it gets hydrolysed by its own water of crystallisation.
Anhydrous MgCl2 is fused with anhydrous NaCl and CaCl2 and electrolysed at 700ºC.
At Cathode : 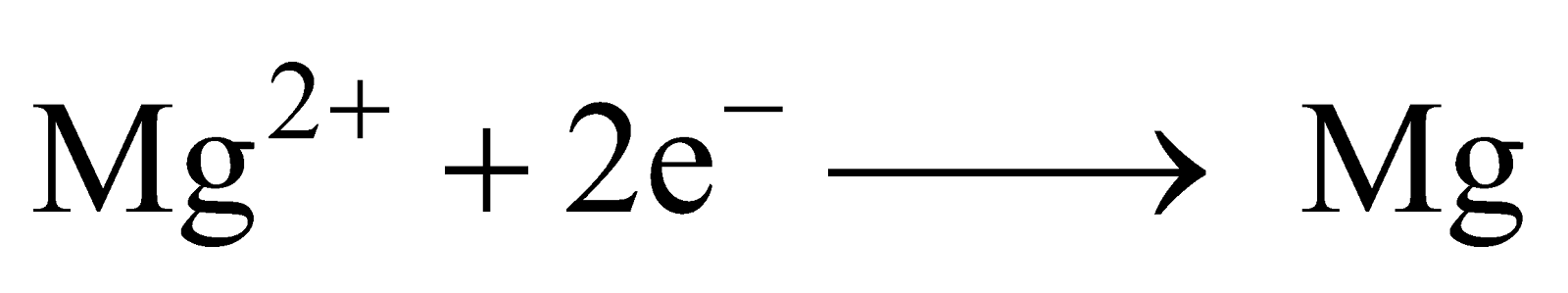
At anode: 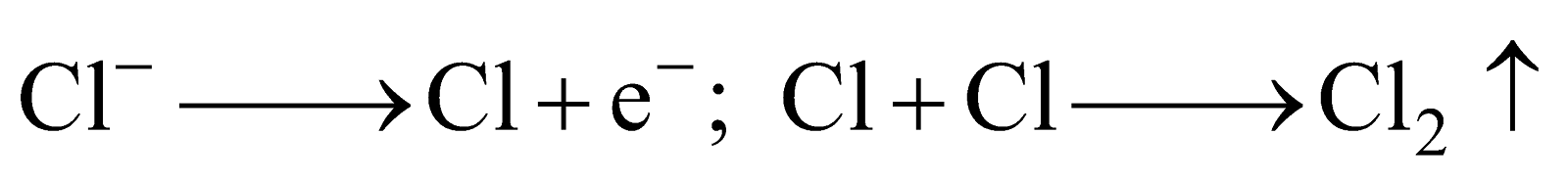
USES OF MAGNESIUM
- Mg being a light metal forms alloys with Al and Zn which are used in aircraft construction. e.g., elektron (95% Mg + 5% Zn) used in construction of aircraft, magnalium (1-15% Mg + 85-99% Al) used in construction of aircraft and light instruments.
- Magnesium powder is used in flash bulbs used in photography.
COMPOUNDS OF MAGNESIUM
MAGNESIUM OXIDE, MAGNESIA, MgO
With MgCl2, it forms a mixture of composition MgCl2.5MgO.xH2O which is known as Sorel’s cement or magnesia cement.
MAGNESIUM HYDROXIDE, MILK OF MAGNESIA, Mg(OH)2
Its aqueous suspension is used in medicine as an antacid.
MAGNESIUM SULPHATE OR EPSOM SALT, MgSO4.7H2O
It shows isomorphous nature with ZnSO4.7H2O, deliquescence and efflorescence. It is used as a purgative in medicine and as a stimulant to increase the secretion of bile.
MAGNESIUM CARBONATE, MAGNESITE, MgCO3
- It dissolves in water in the presence of CO2.
- Its 12% aqueous solution is known as fluid magnesia and is used as an antacid, laxative and in toothpastes.
MAGNESIUM CHLORIDE, MgCl2.6H2O
It is a deliquescent, white crystalline solid.
METALLURGY OF CALCIUM
OCCURRENCE AND IMPORTANT MINERALS
- It is an important constituent of bones and teeth (as calcium phosphate), sea shells and corals (as calcium carbonate).
- The important minerals are
- Limestone, marble, chalk or calcite, CaCO3
- Dolomite, MgCO3.CaCO3
- Gypsum, CaSO4.2H2O
- Fluorspar, CaF2
- Anhydrite, CaSO4
- Hydroxyapatite, 3Ca3(PO4)2.Ca(OH)2
- Phosphorite, Ca3(PO4)2.
EXTRACTION
It is extracted by the electrolysis of a fused mixture of calcium chloride and calcium fluoride (lowers the fusion temperature of the electrolyte).
USES OF CALCIUM
- It is used to remove air from vacuum tubes, sulphur from petroleum and oxygen from molten steel.
- It is used as a reducing agent in the extraction of such metals from their oxides where carbon is ineffective.
COMPOUNDS OF CALCIUM
CALCIUM OXIDE, QUICK LIME, BURNT LIME, LIME, CaO
PREPARATION
By the thermal decomposition of calcium carbonate.
PROPERTIES
- It is a basic oxide.
- Its aqueous suspension is known as slaked lime Ca(OH)2.
- On heating with ammonium salts it gives ammonia
- It reacts with carbon to form calcium carbide.
- It is used as basic flux, for removing hardness of water for preparing mortar (CaO + Sand + Water).
CALCIUM HYDROXIDE, SLAKED LIME, LIME WATER, Ca(OH)2
PREPARATION
By dissolving quick lime in water.
PROPERTIES
- Its suspension in water is known as milk of lime.
- It gives CaCO3 (milky) and then Ca(HCO3)2 with CO2.
- It reacts with Cl2 to give bleaching powder CaOCl2.
CALCIUM CHLORIDE, CaCl2.6H2O
- It is a deliquescent solid which is a by-product of Solvay’s process.
- Fused Calcium chloride is a good dessicant (drying agent), but it can not be used to dry alcohol or ammonia as it forms addition product with them.
CALCIUM CARBONATE, LIMESTONE, MARBLE, CHALK, SLATE, CALCITE, CaCO3
PREPARATION
By passing CO2 through lime water.
PROPERTIES
It is insoluble in H2O but dissolves in the presence of CO2, due to the formation of calcium bicarbonate.
GYPSUM, CALCIUM SULPHATE DIHYDRATE, CaSO4.2H2O
- It is naturally occurring calcium sulphate and also known as alabaster.
- On heating at 390K, it gives plaster of paris.
- It is added to cement to slow down its rate of setting.
PLASTER OF PARIS, CALCIUM SULPHATE HEMIHYDRATE, CaSO4.1/2 H2O
- When it is mixed with water, it forms first a plastic mass which sets into a solid mass with slight expansion due to rehydration and its reconversion into gypsum.
- On heating at about 200ºC, it also forms dead burnt plaster of paris (it has no tendency to set).
CALCIUM CARBIDE OR CALCIUM ACETYLIDE, CaC2
PREPARATION
By heating a mixture of quick lime (CaO) and powdered coke in an electric furnace at 3300K.
PROPERTIES
- It reacts with water to form acetylene.
- When heated with nitrogen, it forms calcium cyanamide which on reaction with steam under pressure gives NH3.
- Nitrolim (a mixture of calcium cyanamide and carbon) is used as a fertilizer.
BLEACHING POWDER, CALCIUM HYPOCHLORITE, CHLORIDE OF LIME, CaOCl2
PREPARATION
By passing a current of chlorine over dry slaked lime.
MANUFACTURE
The manufacture of bleaching powder is carried out in (i) Hasenclever plant or (ii) Bachmann's plant
PROPERTIES
- It is a mixture (mixed salt) of calcium hypochlorite (Ca.(OCl)2.4H2O) and basic calcium chloride (CaCl2.Ca(OH)2.H2O).
- Its aqueous solution gives Ca2+, Cl– and OCl– ions.
- With dil. H2SO4, it gives nascent oxygen which causes its oxidising and bleaching power.
- With excess of dil. H2SO4 (or CO2), it forms Cl2 known as available chlorine.
The average percentage of available chlorine is 35 - 40%. Theoretically it should be 49%, which diminishes on keeping the powder due to following change 
Available chlorine is estimated by
- Arsenite method (Penot's method)
- Iodometric method (Bunsen and Wagner's method)
- It gives O2 in presence of catalyst COCl2.
USES
It is used for bleaching, as disinfectant and germicide in sterilization of water, for making wool unshrinkable and in the manufacture of Chloroform.
CEMENT
Cement is essentially a mixture of complex silicates and aluminates of Ca containing less than 1.0% free lime and some gypsum (CaSO4.2H2O)
COMPOSITION
An approximate composition is as follows :
Lime
|
CaO
|
60-69%
|
62%
|
Silica
|
SiO2
|
17-25%
|
22%
|
Alumina
|
Al2O3
|
3-8%
|
7.5%
|
Magnesia
|
MgO
|
1-5%
|
2.5%
|
Iron oxide
|
Fe2O3
|
0.5-5%
|
2.5%
|
Sulphur trioxide
|
SO3
|
1-3%
|
1.5%
|
Sodium oxide
|
Na2O
|
0.3-1.5%
|
1.0%
|
Potassium oxide
|
K2O
|
0.3-1.5%
|
1.0%
|
Ratio of Silica and alumina
Ratio of CaO and 6(SiO2 + Al2O3 + Fe2O3)
White Cement : It does not contain ferric oxide
PROCESS
Two processes are employed (i) Wet process (ii) Dry process
Raw material : Lime and Clay
MANUFACTURE
Clay + Lime ➝ Cement clinker ➝ Cement
Gypsum regulates the setting time
SETTING OF CEMENT
When mixed with water, the cement forms a gelatinous mass sets to hard mass when three dimensional cross links are formed between ... Si-O-Si---and ---Si-O-Al--- chains.
The reactions involved in the setting of cement are :
- Hydration : Hydration of 3CaO.Al2O3 and 2CaOSiO2 forming colloidal gel.
- Hydrolysis : Hydrolysis of 3CaOAl2O3 and 3CaO.SiO2 forming precipitates of Ca(OH)2 and Al(OH)3
Fly ash : A waste product of steel industry possess properties similar to cement. It is added to cement to reduce its cost.
Rice Husk : It has high silica content and employed to make cement.










