HYDROGEN
GENERAL INTRODUCTION
Symbol of hydrogen is H. Electronic Configuration 1s1. Hydrogen is the lightest and most abundant element in the universe. It was first prepared by Sir Henry Cavendish by the action of sulphuric acid on Zinc and named by Antoine Lavoisier since it produced water on burning.
(Greek : hydra= water, gennas = maker or producing)
PREPARATION OF DIHYDROGEN
- From cold water : By the action of Na, K, Ca etc.
Al-Hg and Zn-Cu couple decompose water to give nascent hydrogen
Hence Couples constitute better reducing agents
- From hot water : By the action of Mg, Zn, Al etc.
- From steam : By the action of Fe, Sn etc.
- From water : By the action of metallic hydrides of alkali and alkaline earth metals.
- From acids : By the action of dilute acids on Zn, Mg, Fe, etc. placed above hydrogen in electrochemical series.
- From alkalies : By the action Zn, Al, Sn etc.
MANUFACTURE OF HYDROGEN
- From water gas (Bosch process) : By passing water gas mixed with steam over heated catalyst Fe3O4 and Cr2O3 at 450ºC.
The CO2 is removed by dissolving in water under high pressure.
- By Lane’s process : The superheated steam is passed over heated iron at 600 - 800ºC
Iron is regenerated by passing water gas. (H2 + CO)
In actual practice steam is passed over hot iron for 10 - 15 minutes and then water gas is blown over heated oxide for 25 - 30 minutes.
- By electrolysis of water : By the electrolysis of acidified or alkaline water.
At cathode 
At anode 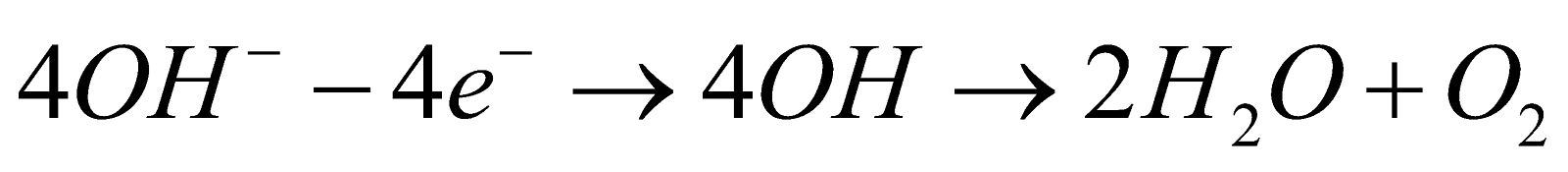
- As by product : When NaOH is manufactured by electrolysis of NaCl in Nelson or Castner Kellner cell hydrogen is obtained as by product.
- From hydrocarbons : By cracking of hydrocarbons
PURE HYDROGEN METHOD OF PREPARATION
- By Uyeno’s method : By the action of Caustic Soda on aluminium.
- By the action of NaH on water
- By treating pure Mg or Al with chemically pure H2SO4 (dil) or HCl
PHYSICAL PROPERTIES
Hydrogen is tasteless, odourless, colourless gas. It is non poisonous but presence of AsH3 makes it poisonous. Its critical temperature is –234.5ºC which makes its liquefaction difficult. It is slightly soluble in water.
ISOTOPES OF HYDROGEN
Hydrogen has three isotopes
Name 1H1 1H2 or D 1H3 or T
Protium Deuterium Tritium
Abundance 99.985% 0.016% Traces (10–15 %)
Deuterium is known as heavy hydrogen.
Tritium is formed in upper atmosphere and is  -radioactive.
-radioactive.
CHEMICAL PROPERTIES
Its chemical properties are
- Reaction with non metals :
- Reaction with metals :
Metals like Fe, Ni and Pd form interstitial or metallic hydrides.
- Reducing property :
- Hydrogenation :
APPLICATIONS OF HYDROGEN
- Manufacture of methyl alcohol :
- Manufacture of ammonia :
- Synthetic petrol : Fisher-Tropsch process
- Manufacture of vegetable ghee : By hydrogenation of oils in presence of Ni.
- To produce low-temperature : It is used as Cryogenic fluid.
- Oxy-hydrogen flame : It produces temperature of 2850ºC and oxy-atomic hydrogen flame produces a temperature of 4000ºC.
- Mixed with Helium - It is used for filling balloons.
FORMS OF HYDROGEN
- Atomic Hydrogen :
It is very reactive and its half life period is 0.33 seconds.
- Occluded Hydrogen : Hydrogen adsorbed by certain metals eg. Pt, Pd, Fe, Ni etc is known as occluded hydrogen. One volume of finely divided metals adsorb the following volumes of hydrogen.
Palladium black 870, Platinum 49.5; Gold 46.3, Iron 15.7, Copper 4.5, Aluminium 2.7.
- Nascent hydrogen : Freshly prepared hydrogen is known as nascent hydrogen and is more reactive than ordinary hydrogen. It causes the reduction of certain compounds which is not possible with ordinary hydrogen.
- Ortho and Para hydrogen : The nucleus of the hydrogen atom also spins like a top. When in hydrogen molecule, the nuclear spins are in the same direction it is known as ortho hydrogen and when the nuclear spins are in the opposite direction it is known as para hydrogen. The two electrons in a hydrogen molecule always spin in opposite direction. At room temperature hydrogen consists of 75% ortho and 25% para. At low temperature more para is present.
- Transportation of hydrogen : It is transported in the form of Hydrolith (CaH2) or ammonia (NH3). Ammonia is cracked by passing over heated catalysts yielding a mixture of hydrogen (75%) and N2 (25%).
HYDRIDES
Binary compounds of hydrogen and other elements are called hydrides. Hydrides are classified into the following four classes.
- Saline or ionic hydrides.
- Molecular or covalent hydrides.
- Metallic or interstitial hydrides.
- Polymeric hydrides.
- Saline or ionic hydrides : These are formed by elements of group 1, 2 (Except Be and Mg) and lanthanides by heating the metal in hydrogen.
These are white colourless solids (crystalline) having high mpt. and bpt. easily decomposed by water, alcohol, CO2 or SO2.
They are strong reducing agents. Alkali metal hydrides are used for making LiAlH4, NaBH4 etc and for removing last traces of water from organic compound.
- Molecular or covalent hydrides : These are formed by 4th, 5th, 6th, 7th group elements and boron by sharing electrons with hydrogen atoms. eg.: NH3, HCl, B2H6, AsH3 . These are non electrolytes and are usually gases or liquids.
- Metallic or interstitial hydrides : The transition elements and rare earth metals combine with hydrogen to give interstitial hydrides. They exhibit metallic properties and are powerful reducing agents. They are non stoichiometric compounds and their composition varies with temperature and pressure. eg. LaH2.76, TiH1.73
- Polymeric hydrides : These are solids containing molecules, linked together in two or three dimensions by hydrogen bridge bonds. e.g.: (BeH2)n, (MgH2)n and (AlH3)n
HYDROGEN PEROXIDE
Discovery : French chemist Thenard 1818.
Occurrence : Traces in air, rain and plants.
PREPARATION OF HYDROGEN PEROXIDE
- Lab method : From true peroxide by the action of ice cold dil. H2SO4.
(HNO3 is not used since it will oxidise H2O2).
- Merck process : By passing CO2 through a suspension of BaO2 in ice cold water.
MANUFACTURE OF HYDROGEN PEROXIDE
- By electrolysis of 50% ice cold H2SO4
Mechanism :
At anode : 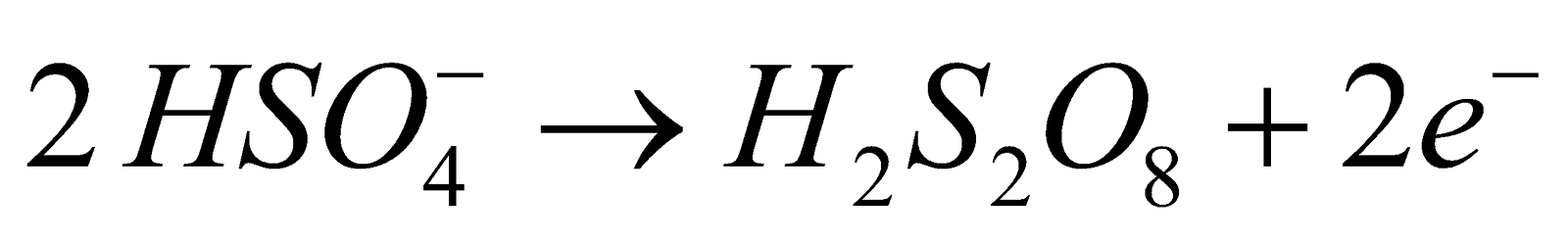
At cathode : 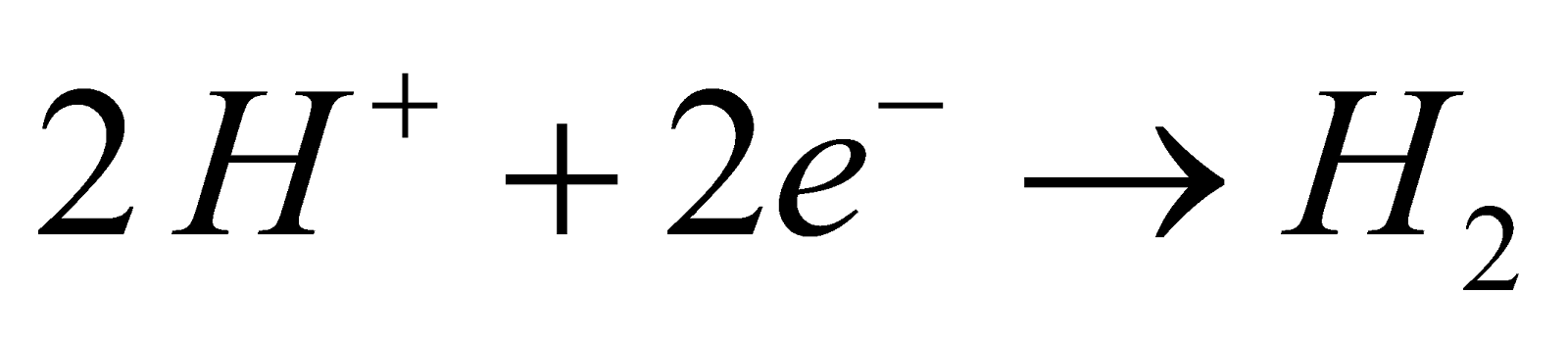
- Auto oxidation : Most recent method used in America. In this method the anthraquinone is reduced to anthraquinol by dissolving in an organic solvent and passing hydrogen in presence of Pd. On frothing, the anthraquinol derivative with air, 20% solution of H2O2 is obtained and anthraquinone is regenerated.
- By electrolysis of ammonium sulphate solution and sulphuric acid. When aqueous solution of ammonium sulphate and sulphuric acid in equimolar proportion is electrolysed at low temperature ammonium persulphate is formed. The latter on distillation with sulphuric acid gives 30% solution of hydrogen peroxide.
At anode : 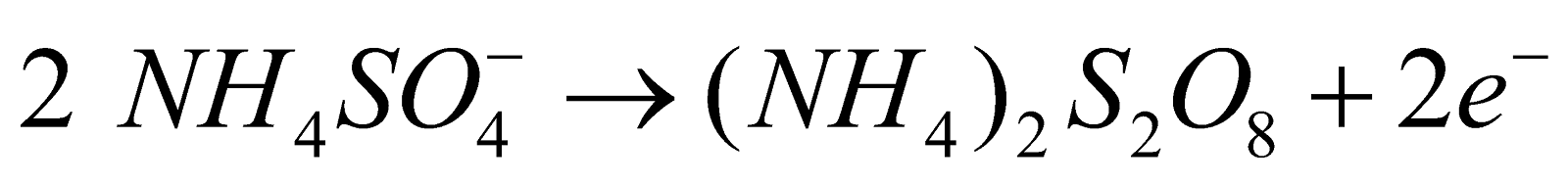
At cathode : 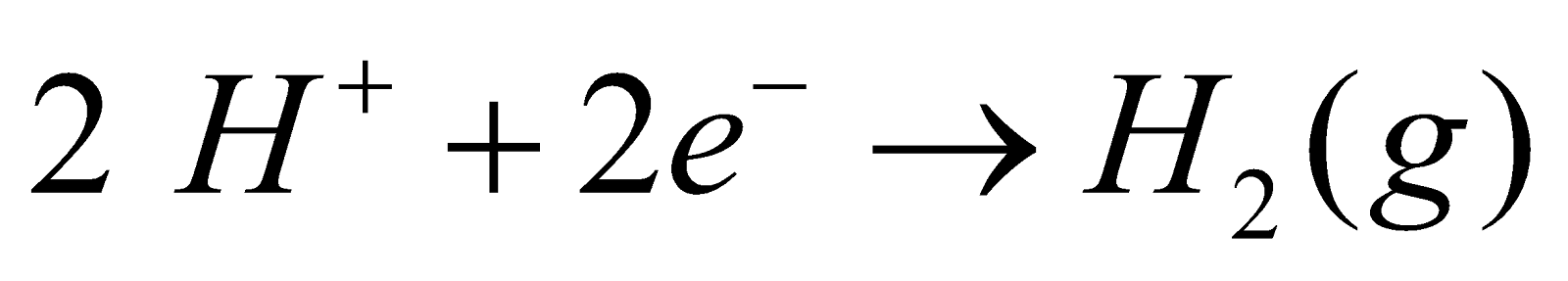
CONCENTRATION OF HYDROGEN PEROXIDE
It is very carefully concentrated since it decomposes on heating or on standing.
Decomposition is catalysed by Cu, Ag, Pt, Co, Fe, MnO2 etc.
The methods employed for concentration are
- Evaporation : By careful evaporation of solution on a water bath (50% H2O2 is obtained).
- Dehydration in vacuum desicator : The 50% H2O2 is dehydrated in a vacuum desicator in presence of conc. H2SO4 when 90% H2O2 is obtained.
- Vacuum distillation : The 90% H2O2 obtained in step (ii) is distilled under reduced pressure to get 100% H2O2
- Cooling : The traces of water left are removed by freezing in a freezing mixture when crystals of hydrogen peroxide separate out.
STRENGTH OF HYDROGEN PEROXIDE
The strength of hydrogen peroxide is indicated in terms of the volume of oxygen at NTP that 1 volume of H2O2 gives on heating. For example “20 volume H2O2 means 1 volume of H2O2 at NTP will give 20 volume of oxygen. The normality and percentage strength of H2O2 can be calculated as follows
2 × 34 = 68 g 1 mole O2 = 22.4 litres of O2 at NTP
22.4 litres of O2 at NTP are evolved from 68g of H2O2
x litres of O2 at NTP would be evolved from 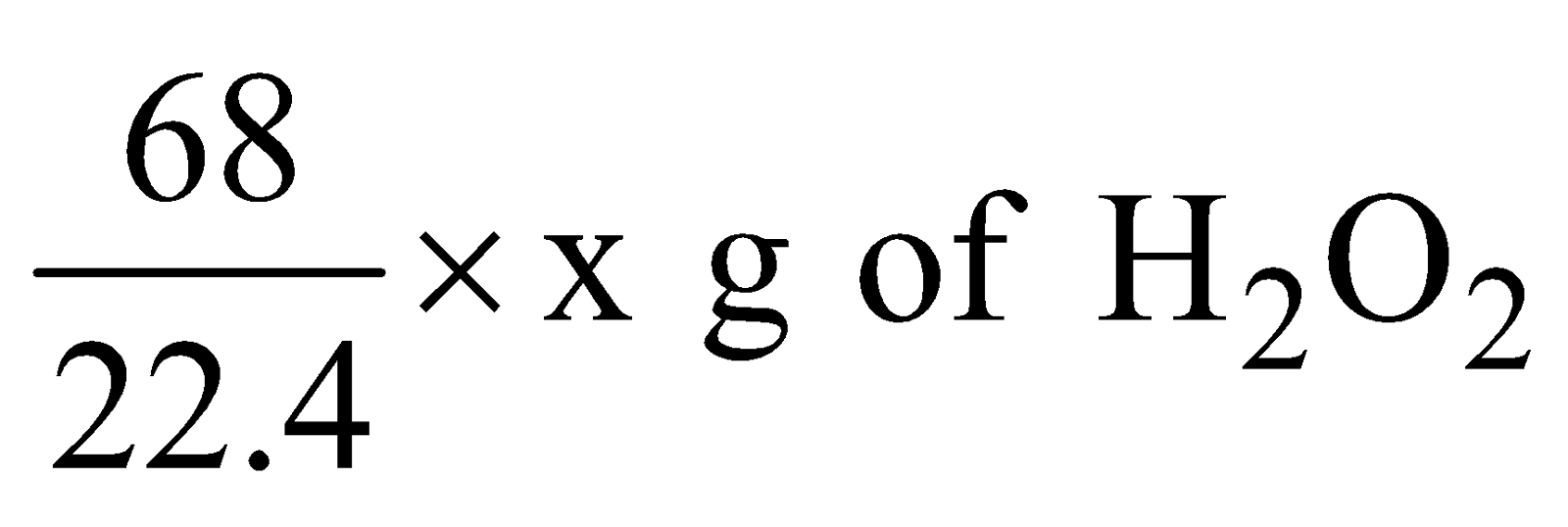
where x is volume strength of H2O2
Hence strength of x volume of H2O2 =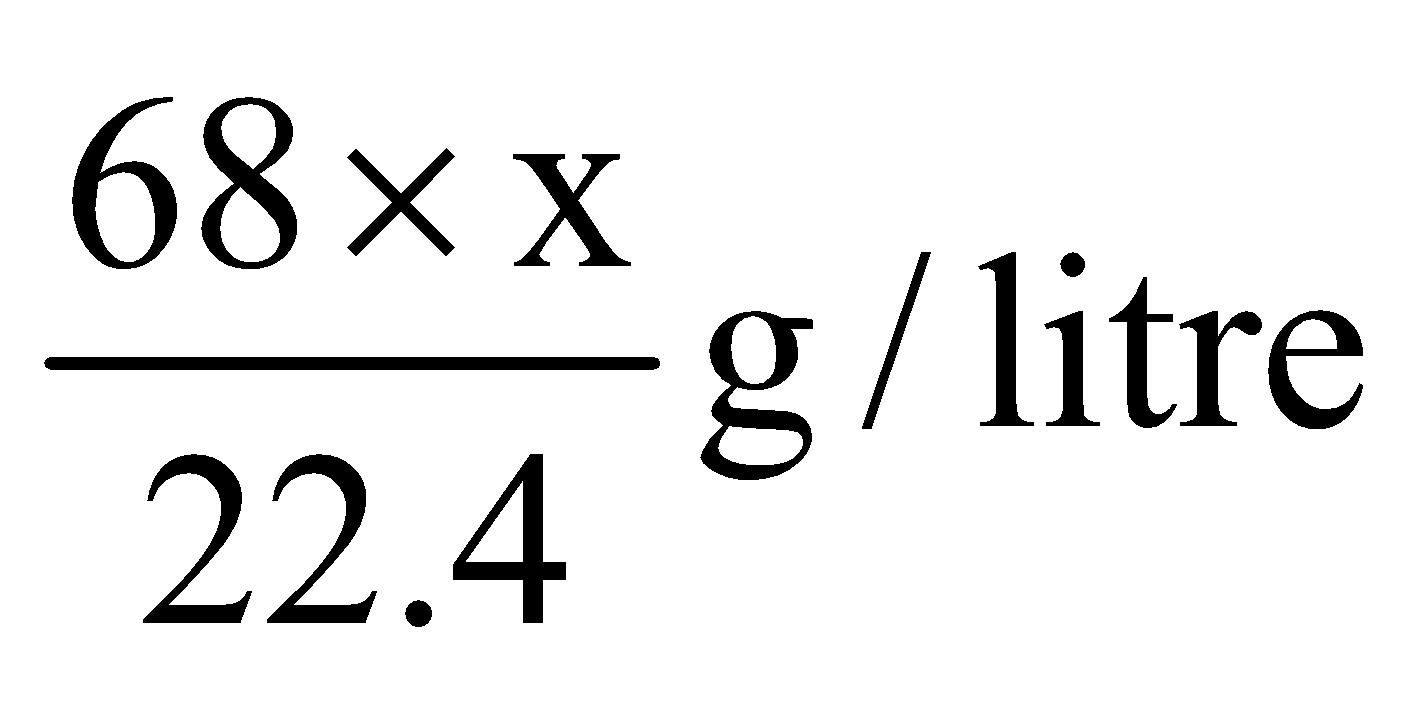
Again, Strength (g/l) = Equivalent weight × Normality
where 17 is the equivalent weight of H2O2
STORAGE OF HYDROGEN PEROXIDE
It is stored in presence of traces of alcohol, acetanilide or sodium pyrophosphate which slow down the rate of decomposition of hydrogen peroxide.
CHEMICAL PROPERTIES OF HYDROGEN PEROXIDE
- Acidic nature : It is weakly acidic in nature and pure hydrogen peroxide turns blue litmus into red. (Ka = 1.57 × 10–12 at 293 K). It ionises in two steps
H2O2 H+ +
H+ + 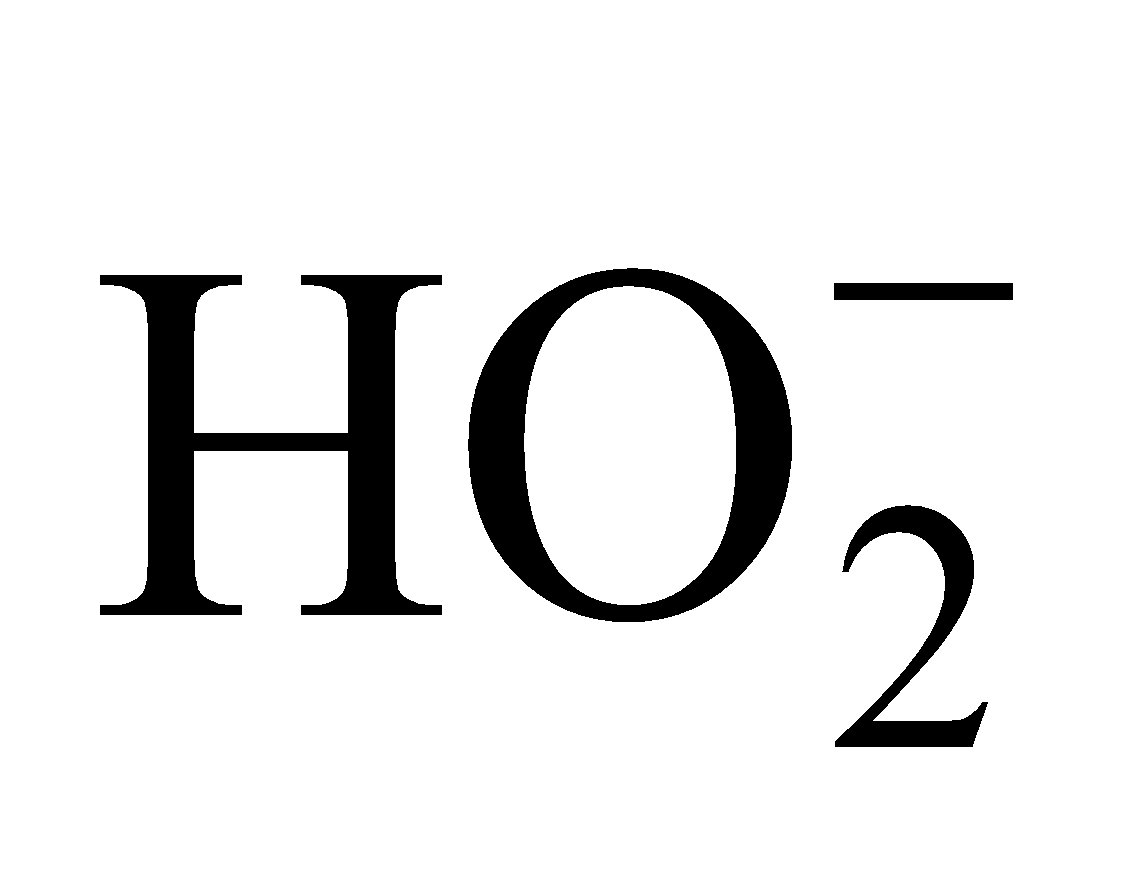
Hence it forms two series of salts eg. NaHO2 sodium hydroperoxide and Na2O2 (Sodium peroxide)
- Oxidising agent : It is strong oxidising agent in acidic as well as in basic medium.
or
In basic medium
- Reducing agent :
- In acidic medium
- In basic medium
- Bleaching properties : Its bleaching action is due to oxidation reaction.
STRUCTURE OF HYDROGEN PEROXIDE
It is represented as follows
TEST OF HYDROGEN PEROXIDE
- It liberates I2 from acidified KI
- Perchromic acid :
- With titanic sulphate it gives orange red pertitanic acid
- Black lead sulphide turned white
USES OF HYDROGEN PEROXIDE
It is used as a bleaching agent, disinfectant, source of power (90% H2O2 as fuel in submarines, rockets and helicopters), in restoration of old paintings in which lead oxide is used as white paint.
WATER
Water is one of the most abundant substances in nature. The 4/5th of the earth surface is covered with water.
SOURCES OF WATER
The sources of water are
- Surface water
- Flowing water - streams and rivers
- Still water - ponds, lakes and reservoirs
- Underground water - water from wells
- Rain water
- Sea water
TYPES OF IMPURITIES PRESENT IN WATER
- Dissolved impurities
- Inorganic salts eg. : Ca2+, Mg2+, Fe2+, Al3+, Na+, K+ traces of Zn2+ and Cu2+ (cations) and Cl–, F– etc. (anions)
- Gases eg.: CO2, N2, O2, oxides of nitrogen, H2S etc.
- Organic salts
- Suspended impurities :
- Inorganic : eg.: sand and clay
- Organic : eg.: animal matter, vegetable etc.
- Colloidal impurities : Finely divided clay, Al(OH)3, Fe(OH)3 colouring matter etc.
- Bacterial impurities : Micro-organisms and bacteria
Effect of impurities : The impurities effect the followings
- Colour
- Taste
- Hardness
- Alkalinity
- Turbidity
- Odour
SOFT AND HARD WATER
The water which produces large amount of lather with soap is known as soft water and which forms a scum with soap is known as hard water.
TYPES OF HARDNESS OF WATER
- Temporary hardness : It is due to the presence of bicarbonates of calcium or magnesium or both.
- Permanent hardness : It is due to the presence of chlorides and sulphates of calcium and magnesium.
REMOVAL OF TEMPORARY HARDNESS
It can be achieved by following methods
- By boiling : The soluble bicarbonates are converted into insoluble carbonates.
- By Clark’s process : By adding lime water or milk of lime
REMOVAL OF PERMANENT HARDNESS
- By adding washing soda : The calcium or magnesium salts are precipitated as carbonates
- By adding Caustic Soda : The temporary and permanent hardness can be removed by adding caustic soda
- By adding Sodium phosphate (Na3PO4) : The phosphates of calcium and magnesium are precipitated
- Calgon process : Calgon is sodium hexa metaphosphate. The water is passed through the bed of calgon the Ca2+ and Mg2+ form soluble complex.
Water becomes free from Ca++ and Mg++ ions.
- Permutit process : Permutit is hydrated Sodium aluminium silicate Na2 Al2 Si2 O8.xH2O. It exchanges its sodium ions for divalent ions such as Ca2+ and Mg2+.
Permutit when fully exhausted can be regenerated by treating with 10% solution of sodium chloride
It is most efficient method to get water with zero degree hardness.
- By synthetic resins : They are of two types :
- Cation exchange resins : These are giant molecules containing sulphonic acid group (–SO3H). It is first changed into sodium salt and has the general formula
The hard water is passed through it when Ca2+ and Mg2+ are exchanged and removed.
The resins like permutit can be regenerated with a solution of common salt.
- Anion exchange resins : These are also giant molecules and can exchange anions. They contain an amino group.
Anion exchange resin Exhausted anion exchange resin.
The water is first passed through cation resins and then through anion resins and pure distilled water is obtained.
DEGREE OF HARDNESS
The hardness of water is expressed in terms of ppm of calcium carbonates.
1CaCO3 ≡ 1MgCl2 ≡ 1MgSO4 ≡ 1CaCl2 ≡ 1CaSO4
100ppm 95ppm 120ppm 111ppm 136ppm
HYDRATES
The substances (salts) containing water molecules are called hydrates. These are of three types:
- Cationic hydrates : When water molecules are held by cations by coordinate bonds, the hydrates are known as Cationic hydrates. eg. : MgCl2.6H2O, CaCl2.6H2O, etc.
- Anionic hydrates : In this case the water molecules are held by anions as well as cations by coordinate bonds. eg. : MgSO4.7H2O, CuSO4.5H2O.
- Lattice hydrates : The water molecules occupy the lattice sites e.g. : Na2CO3.10H2O, K2SO4. Al2(SO4)3. 24H2O. On heating the water molecules are lost and substances change to powder form.
HEAVY WATER/DEUTERIUM OXIDE (D2O)
It was discovered by Urey, who showed that ordinary water contains one part of heavy water in 6,000 parts of it.
PREPARATION
It is prepared by exhaustive electrolysis of water containing alkali with nickel electrodes. About 20 litres of ordinary water gives 0.5 ml of heavy water.
PROPERTIES
Heavy water is a colourless, odourless, tasteless mobile liquid. Its physical properties in comparison to ordinary water are as follows:
Physical constants : D2O H2O
Melting point 276.8K 273K
Boiling point 374.4K 373K
Sp. gravity at 20º C 1.106 0.998
Temperature of max. density 284.6K 277K
Specific heat at 20º C 1.018 1.000
Viscosity at 293 K 14.2 10.87
Surface tension 67.8 72.8
Latent heat of vaporisation 2330 kJkg–1 2255 kJkg–1
Dielectric Constant 82 80.5
Solubility of NaCl at 20ºC 30.5% 35.9%
Refractive index 1.328 1.33
CHEMICAL PROPERTIES
- Electrolysis :
- Reaction with Na :
- Reaction with acid oxides :
- Reaction with metallic carbides :
- Deuterolysis :
- As water of crystallisation : It gives deutero hydrates CuSO4.5D2O, MgSO4.7D2O, etc.
Theoretically six different types of heavy water are possible
eg. : 
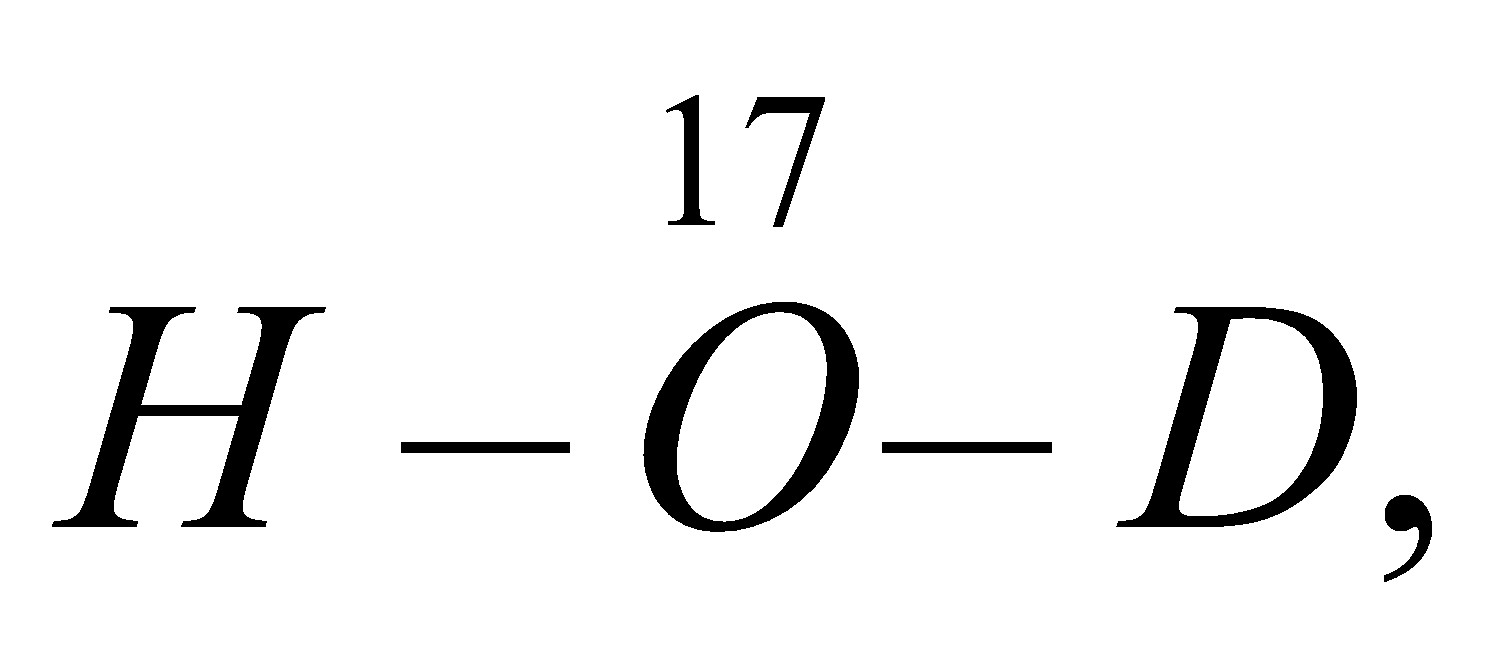

BIOLOGICAL AND PHYSIOLOGICAL EFFECTS
It does not support life, and is injurious to living organism. It checks the growth of plants and animals.
USES
- As a tracer compound
- For production of heavy hydrogen.
- As moderator in nuclear reactors.










