ISOMERISM
ISOMERISM
Berzelius introduced the term Isomer (Gr. Isos=Same, Mers=parts) to different compounds with the same molecular formula and the phenomenon was called Isomerism.
TYPES OF ISOMERISM
There are two main types of Isomerism
- Structural or constitutional isomerism : It is due to difference in the arrangement of atoms within the molecule.
- Stereo isomerism or space isomerism : It is due to different spatial arrangement of some atoms and groups.
TYPES OF STRUCTURAL ISOMERISM
CHAIN ISOMERISM
This is due to difference in the structure of the carbon chains. Examples.
FUNCTIONAL ISOMERISM
This is due to difference in the functional groups
C2H5OH 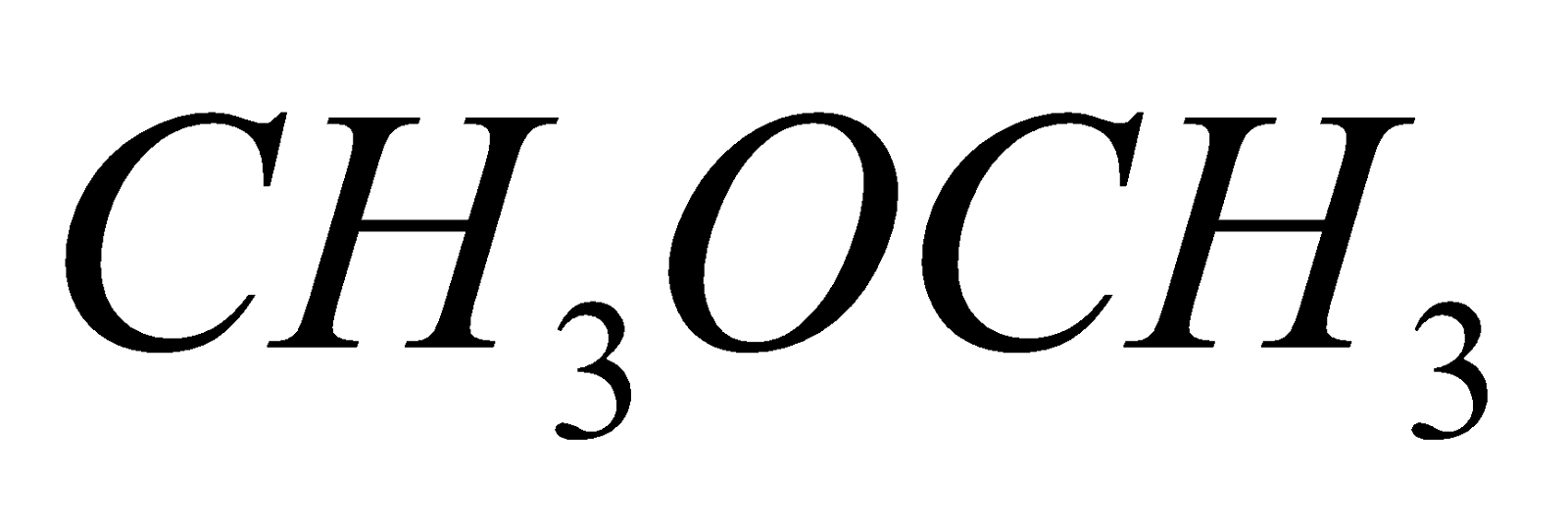
ethyl alcohol dimethyl ether
CH3COOH HCOOCH3
Acetic acid Methyl formate
Propionaldehyde Acetone.
POSITION OR REGION ISOMERISM
This is due to difference in the positions of the same functional groups
n- Propyl alcohol Iso-Propyl alcohol
Position Isomers are also known as regiomers.
METAMERISM
This is due to different alkyl groups attached to the same functional group.
RING CHAIN ISOMERISM
Cyclic/acyclic Isomerism
TAUTOMERISM
(Tauto = Same, Mers = Parts)
It is due to the presence of a mobile atom in the molecule and the same substance behaves in such a way as if it is a mixture of two or more compounds. Further we have:-
DYAD SYSTEM
When the mobile atom oscillates between two adjacent atoms eg:-
Hydrocyanic acid Iso-hydrocyanic acid
TRIAD SYSTEM
When the mobile atom oscillates between atoms one position ahead eg.
93% Keto form 7% Enol form
Aceto acetic ester Aceto acetic ester
Aceto acetic ester reacts with HCN, NH2OH, C6H5NHNH2 showing the properties of a ketone and also reacts with CH3COCl, PCl5, Na showing the properties of OH group.
It gives colour change with 1% FeCl3 a characteristic test of  (enol group)
(enol group)
There exists an equilibrium between the two forms which is dynamic in nature.
Acetone 99.5% Acetone (enol form) 0.5%
Acetyl acetone (keto form) Acetyl acetone (enol form)
Triad system containing nitrogen
It dissolves in NaOH on account of aci form.
Tautomeric form which is less stable is called labile form.
TYPES OF STEREO ISOMERISM
- Optical Isomerism
- Geometrical Isomerism and
- Conformational Isomerism
OPTICAL ISOMERISM
The compounds having the same molecular formula, the same structural formula but different behaviour towards the plane polarised light are known as Optical Isomers.
Terminology used in optical isomerism
- Plane polarised light : Light having vibrations restricted to one plane only is called plane polarised light.
- Optically active compounds : The compounds capable of rotating the plane of polarisation of plane polarised light are known as optically active compounds.
- Optical activity : It is the ability of a substance to rotate the plane of polarisation of plane polarised light.
- Dextrorotatory compounds (d or +) : The compounds which rotate the plane of polarisation of plane polarised light towards the right hand side are called dextro rotatory.
- Laevo rotatory compounds (l or –) : The compounds which rotate the plane of polarisation of plane polarised light towards the left hand side are called laevo rotatory.
- Specific rotation : The rotatory power of optically active compounds is compared in terms of specific rotation.
Specific rotation =
D corresponds to D line of Sodium light ( = 5893Å)
= 5893Å)
t corresponds to temperature
Rotation is observed and measured with a polarimeter
The specific rotation varies with light l and temperature.
The specific rotation varies with light l and temperature.
- Optical activity due to crystalline structure : Some compounds are optically active only in crystalline form. They loss their optical activity when dissolved or fused e.g. Quartz.
- Optical activity due to molecular structure : Some compounds are optically active in solid as well as in solution e.g. tartaric acid. Hence their optical activity is due to their molecular structure which remains the same in solid form and in solution.
- Asymmetric carbon atom : A carbon atom attached to four different atoms and groups is called asymmetric carbon atom. e.g.
.
- Chirality : If the mirror image of the molecule is different from the molecule it is said to be a chiral molecule. In such case if one configuration of the molecule is placed above its mirror image configuration, the similar atoms and groups do not fall over each other and the configurations are said to be nonsuperimposable.
If object and mirror image configurations are superimposable (similar atoms and groups fall over each other) the molecule is said to be "achiral”.
Chirality is the necessary condition for a compound to be optically active.
Enantiomers : Pairs of nonsuperimposable mirror images are called enantiomers.
FISCHER PROJECTIONS
Fischer projections are drawn with a cross, with chiral atom at the centre of the cross.
The horizontal line represents wedges (bonds) coming out of the plane of the paper. The vertical line represents dashed lines (bonds) in the plane of the paper (Bow-tie convention). The carbon chain is drawn along the vertical line of the projection with most highly oxidised carbon substituent at the top. Fischer projections are very useful to determine chirality of a compound.
LEBEL AND VAN'T HOFF’S THEORY ABOUT OPTICAL ISOMERISM
The tetrahedral structure of a compound containing asymmetric carbon atom (*Cabed) gives two configurations related to each other as object and its mirror image but are non-superimposable.
OPTICAL ISOMERISM OF LACTIC ACID
Racemic Lactic acid : It is an equimolar mixture of d- and l- forms. It is optically inactive due to external compensation of optical rotation. It is present in sour milk. It can be resolved.
Examples of optically active compounds containing one asymmetric C-atom.
Number of optically active forms is given by 2n where n is number of asymmetric C-atoms different in nature.
Resolution : The separation of d- and l- forms present in a racemic mixture is known as resolution.
CONDITIONS FOR CHIRALITY
Absence of:-
- Plane of symmetry
- Centre of symmetry
- Alternating axis of symmetry.
Plane of Symmetry : A plane which divides the molecule in two portions in such a way that one portion is the mirror image of the other eg. Tartaric acid.
It is optically inactive due to internal compensation of optical rotation. It can not be resolved.
Centre of Symmetry : It is a point from which lines, when drawn on one side to meet the groups and produced to an equal distance on the other side of the point will meet the same original groups.
Alternating axis of symmetry : If a molecule is rotated through an angle of  about the axis and then reflected in a plane perpendicular to the axis, gives back the original molecule it is said to possess the n fold alternating axis of symmetry.
about the axis and then reflected in a plane perpendicular to the axis, gives back the original molecule it is said to possess the n fold alternating axis of symmetry.
OPTICAL ISOMERISM OF TARTARIC ACID
It contains two similar asymmetric C–atoms 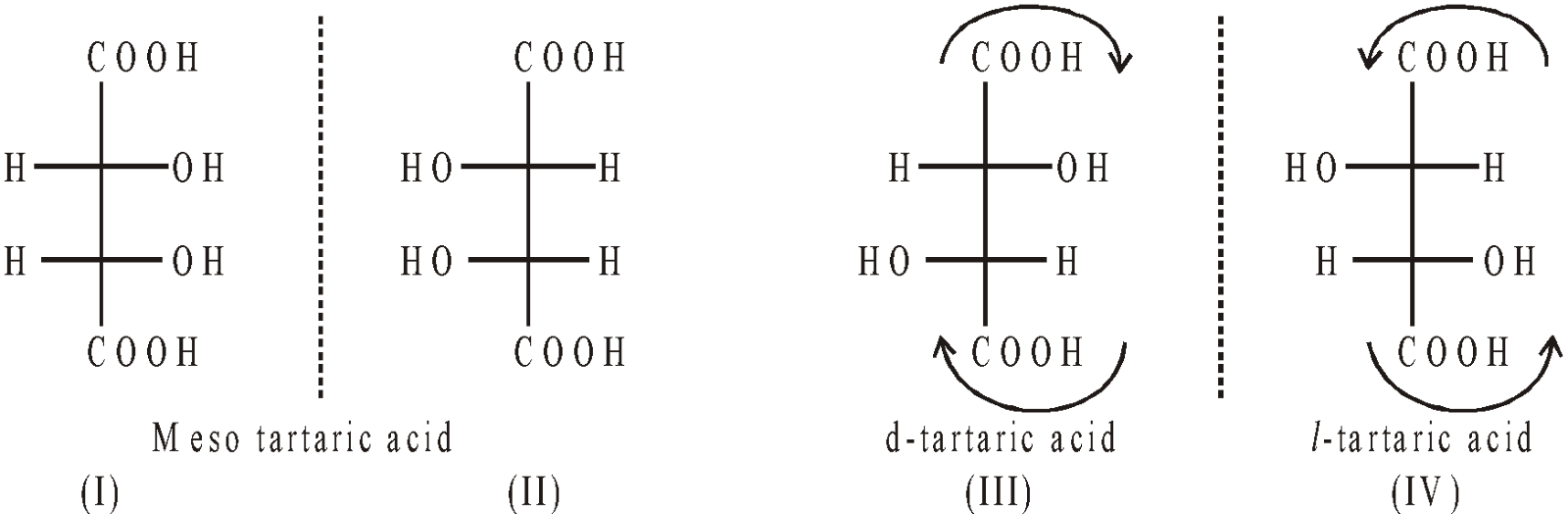
- When the configuration II is rotated through an angle 180° the configuration I is obtained hence they are not enantiomers but represent one single compound.
- d-tartaric acid is obtained from grapes and tamarind. Its mpt is 170°C.
- l-tartaric acid is prepared by resolving racemic acid. Its mpt is 170°C.
- Meso-tartaric acid is obtained by oxidation of maleic acid, heating d-tartaric acid with water at 170°C. Its mpt is 143°C.
- Racemic tartaric acid (dl or ±). It is obtained from Argol. Its mpt is 206°C. It is an equimolar mixture of d and l forms.
- Racemic tartaric acid can be resolved into d and l forms. It is a mixture of two compounds.
- Meso-tartaric acid cannot be resolved. It is a single compound.
OPTICAL ISOMERISM OF THE COMPOUND CONTAINING TWO DISSIMILAR C-ATOMS
Example  ,
, -dibromo cinnamic acid and 2,3-dihydroxy butanoic acid.
-dibromo cinnamic acid and 2,3-dihydroxy butanoic acid.
Let optical rotation due to chiral centre C3 and C2 be A and B and further A > B. In the above case I-II and III-IV are pairs of enantiomers where as I-III; I-IV, II-III, and II-IV are pairs of diastereo-isomers.
DIASTEREO ISOMERS
Stereo isomers which are not mirror images of each other are called diastereo isomers. They have different physical properties (mpt, bpt, solubility) and are often easy to separate by distillation, recrystallisation, chromatography etc.)
The same compound pair are called the meso diastereoisomer (I-II in case of Tartaric acid see above). Most diastereo-isomers are either geometric isomers or compounds with two or more chiral atoms.
ENANTIOMERS
Enantiomers are non superimposable mirror image isomers. They have identical physical properties (bpt, mpt, density etc.) except for their rotation of plane polarised light. They are much more difficult to separate. In nature very often only one enantiomer is produced. Living organisms are one of the best sources of optically active compounds (plants, enzymes, animals, cells etc.).
ASYMMETRIC SYNTHESIS
The synthesis of an optically active compound from optically inactive compound under the influence of an optically active compound without resolution is known as asymmetric synthesis. 
RACEMISATION
The transformation of an optically active isomer under the influence of heat, light or some reagents into an inactive isomer is called racemisation.
WALDEN INVERSION / OPTICAL INVERSION
The conversion of an enantiomer into another is called Walden inversion.
OPTICAL ISOMERISM DUE TO RESTRICTED ROTATION
- Diphenyls :
- Substituted allenes : Unsymmetrically substituted allene (CH2 = C = CH2) are optically active.
Enantiomeric excess (Optical Purity) : It is given by
Optical purity = O.P.
=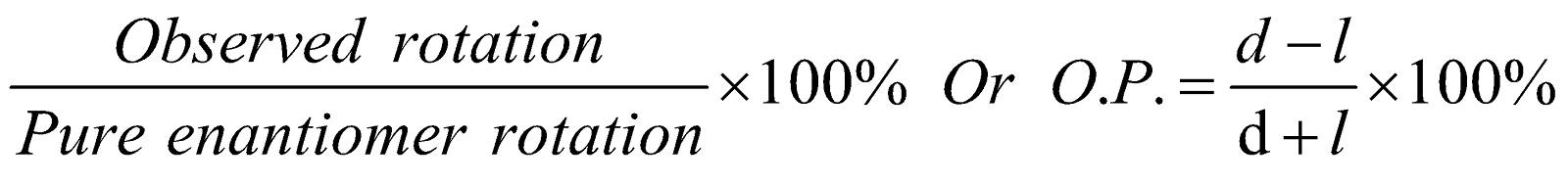
where d and l are ratio of two forms
GEOMETRICAL ISOMERISM
Alkenes with double bonds cannot undergo free rotation and can have different geometrical shapes with two different groups on each end of the double bond. e.g. molecules C2a2b2, C2a2bd or C2abde.
I-II, III-IV and V-VI are pairs of geometrical isomers.
NOMENCLATURE
Cis Isomer : Contains the similar atoms or groups on the same side.
Trans Isomer : Contains the similar atoms or groups on the opposite side.
GEOMETRICAL ISOMERISM OF OXIMES
Aldoximes :
Ketoximes :
GEOMETRICAL ISOMERISM OF AZO COMPOUNDS
Azobenzene :
GEOMETRICAL ISOMERISM IN CYCLO ALKANES
Cyclo alkanes also cannot undergo free rotation.
DETERMINATION OF CONFIGURATION OF GEOMETRICAL ISOMERS
- Physical methods : In general the cis isomer has low mpt, higher bpt, high density higher dipole moment, greater solubility, higher refractive index, higher heat of combustion.
- By Cyclisation :
Hence Maleic acid must be cis isomer.
- By Oxidation :
Hence Maleic must be cis and fumaric must be trans.
E,Z SYSTEM OF NOMENCLATURE FOR GEOMETRICAL ISOMERS
If two high-priority groups are on the same side, the configuration is Z (German, Zusammen = together). If they are on opposite side, the configuration is E (German; entgegen = opposite)
Assignment of Priority : Atoms with higher atomic numbers receive higher priorities
R and S Assignments : By Cahn-Ingold-Prelog. Enantiomers are designated as (R) and (S) according to following rules
- Atoms with higher atomic numbers receive higher priorities.
- When the same atom is bound directly to the chiral carbon, we go to the next atom along the chain.
- Double and triple bonds are treated as if each bond were to a separate atom. e.g.
Thus between –CHO (O, O, H) and –CH2OH (O, H, H) the former will have priority.
- The molecule is drawn in three dimensions in such a way that the bond between the chiral carbon and the lowest priority group heeds back into the paper.
- Draw an arrow from the group of highest priority, to the second, to the third priority group.
- If the arrow is clockwise, the chiral carbon is assigned (R). If the arrow is anticlockwise the chiral carbon is assigned (S).
Example : Alanine
(Always exchange the groups twice to get the same compound. If you exchange the groups once you get the enantiomer).
By using Fischer Projections
Move the group of lowest priority to the bottom.
Molecules with two or more chiral atoms
Hence the compound is (2S, 3R).
is (2S, 3R).










