ENVIRONMENTAL CHEMISTRY
POLLUTION
Any material affecting the life is known as pollutant and the phenomenon is known as pollution. The pollutants may be inorganic, biological or radiological in nature.
Primary pollutants : These are emitted directly from the sources. eg. inorganic gases such as H2S, SO2, CO, NO, HF, NH3 radioactive substances or particulates such as smoke, ash, dust, fumes.
Secondary pollutants : These are formed in the atmosphere by chemical interactions among primary pollutants eg. SO3, NO2, CH4, aldehydes, ketones, nitrates, sulphates, phenols.
Bio-degradable pollutants : These are domestic wastes which are rapidly decomposed by microorganisms.
Non-degradable pollutants : These include chemicals, mercuric salts, lead compounds, pesticides etc.
Natural pollution : It is caused by radioactive substances, volcanic eruptions, forests and mines fires, floods etc.
Artificial pollution : It is caused by industries, thermal plants, automobile exhausts, sewage etc.
ENVIRONMENT
The conditions existing around animal or human life. It is further classified as
- Atmosphere :- The gaseous envelope surrounding the earth.
- Stratosphere : The layer of the earth’s atmosphere above the troposphere and below the mesosphere.
- Troposphere : The lowest region of the atmosphere extending from earth’s surface to the lower boundary of the stratosphere. It contains water vapour and is greatly affected by air pollution.
- Thermosphere : The upper region of the atmosphere above the mesosphere. It is the hottest region (temp upto 1200ºC).
- Mesosphere : The region of the earth’s atmosphere above the stratosphere and below the thermosphere. It is the coldest region (temp. –2 to –92ºC) of atmosphere.
- Exosphere : The uppermost region of atmosphere. It contains atomic and ionic O2, H2 and He.
- Hydrosphere :- The aqueous envelop of the earth eg. oceans, lakes etc.
- Lithosphere :- The solid rocky portion of the earth.
- Biosphere :- The biological envelop which supports the life.
AIR POLLUTION
MAJOR AIR POLLUTANTS
The major air pollutants are following:-
- Carbon monoxide (CO)
It is produced by incomplete combustion of gasoline in motor vehicles, wood, coal, incineration and forest fires. It is treacherous and deadly poisonous gas. It induces headache, visual difficulty coma and death. It blocks the normal transport of oxygen from the lungs to other parts of the body. - Sulphur dioxide (
)
It is produced by petrol combustion, coal combustion, petrol refining and smelting operations.
It hinders the movement of air in and out of lungs. It is particularly poisonous to trees causing chlorosis and dwarfing. In presence of air it is oxidised to which is also irritant.
2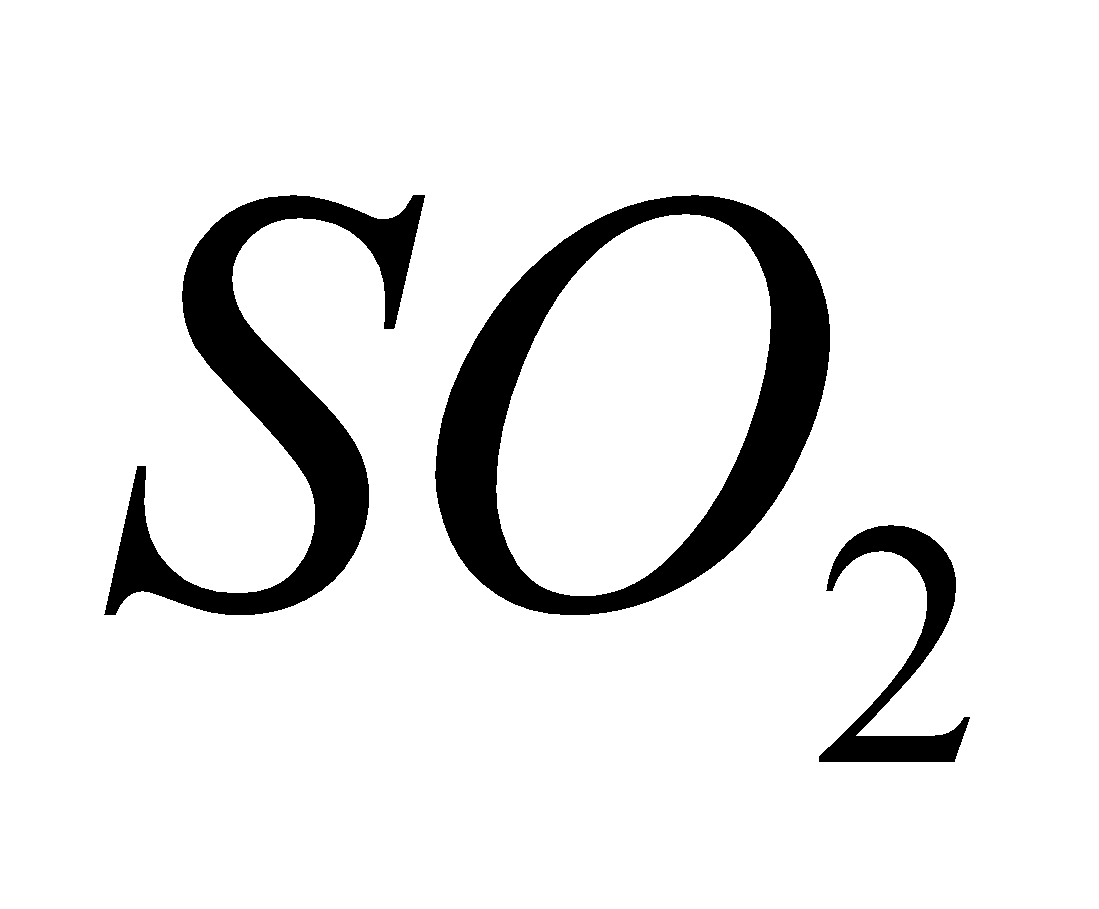 +
+  (air)
(air)  2
2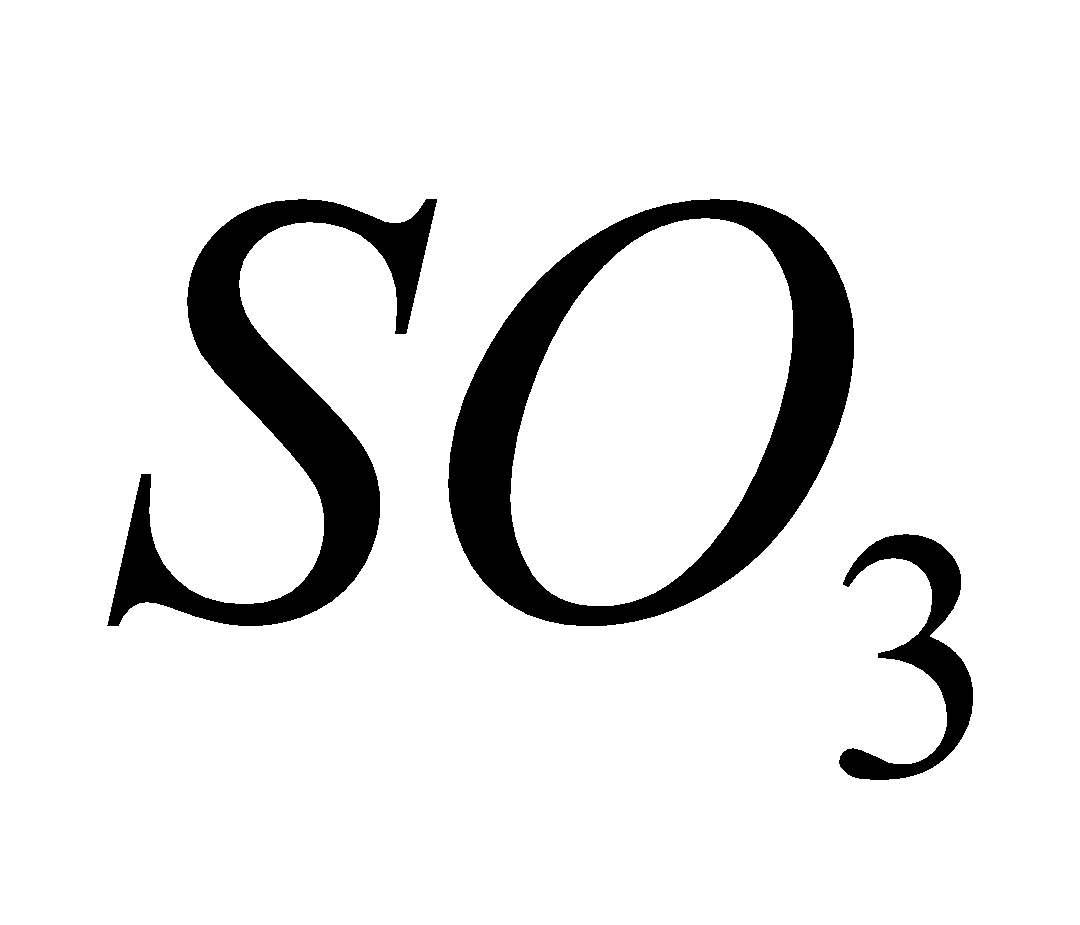
In presence of moisture is converted into highly corrosive sulphuric acid.
It attacks marble, limestone,vegetation, paper and textiles and injurious to human beings.
- Oxides of nitrogen (
and NO)
Source - combustion of coal, gasoline, natural gas, petroleum refining, chemical plants manufacturing explosives and fertilizers, tobacco smoke.
Breathing 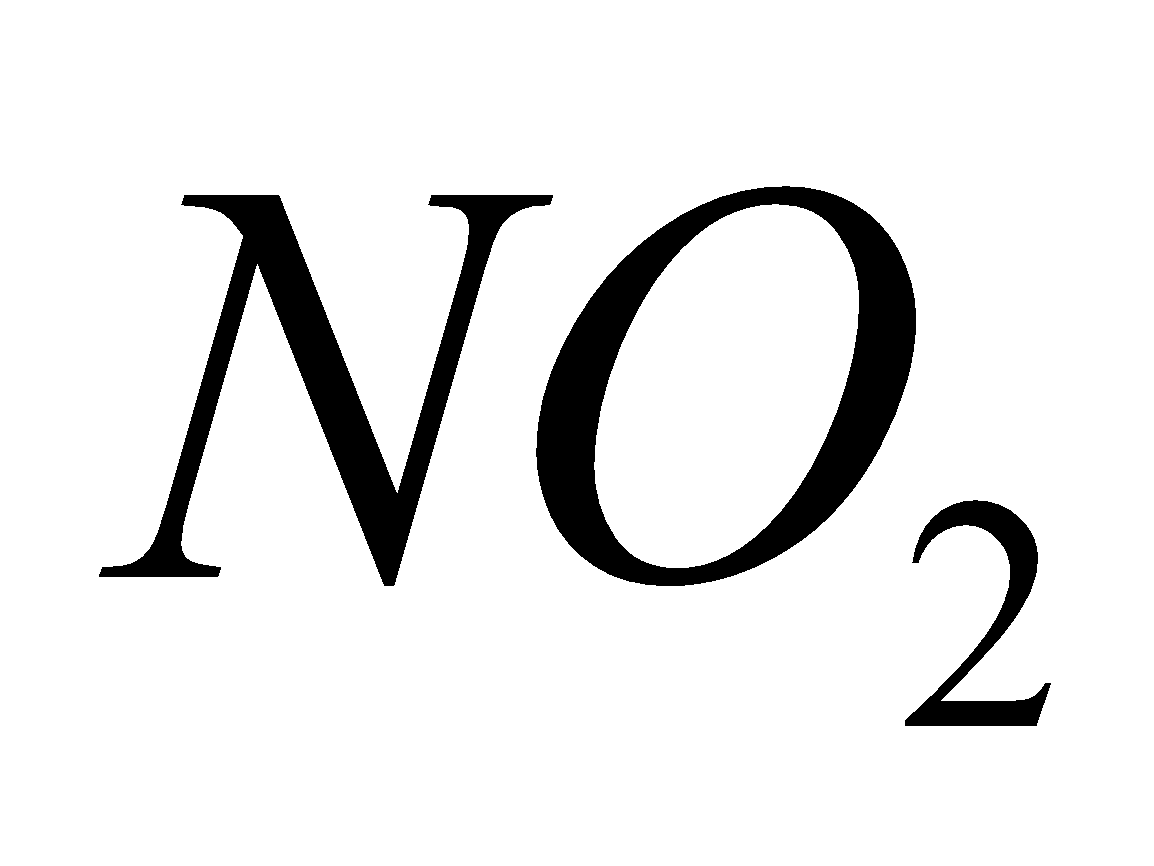 causes chlorosis to plants and chronic lung conditions leading to death.
causes chlorosis to plants and chronic lung conditions leading to death. 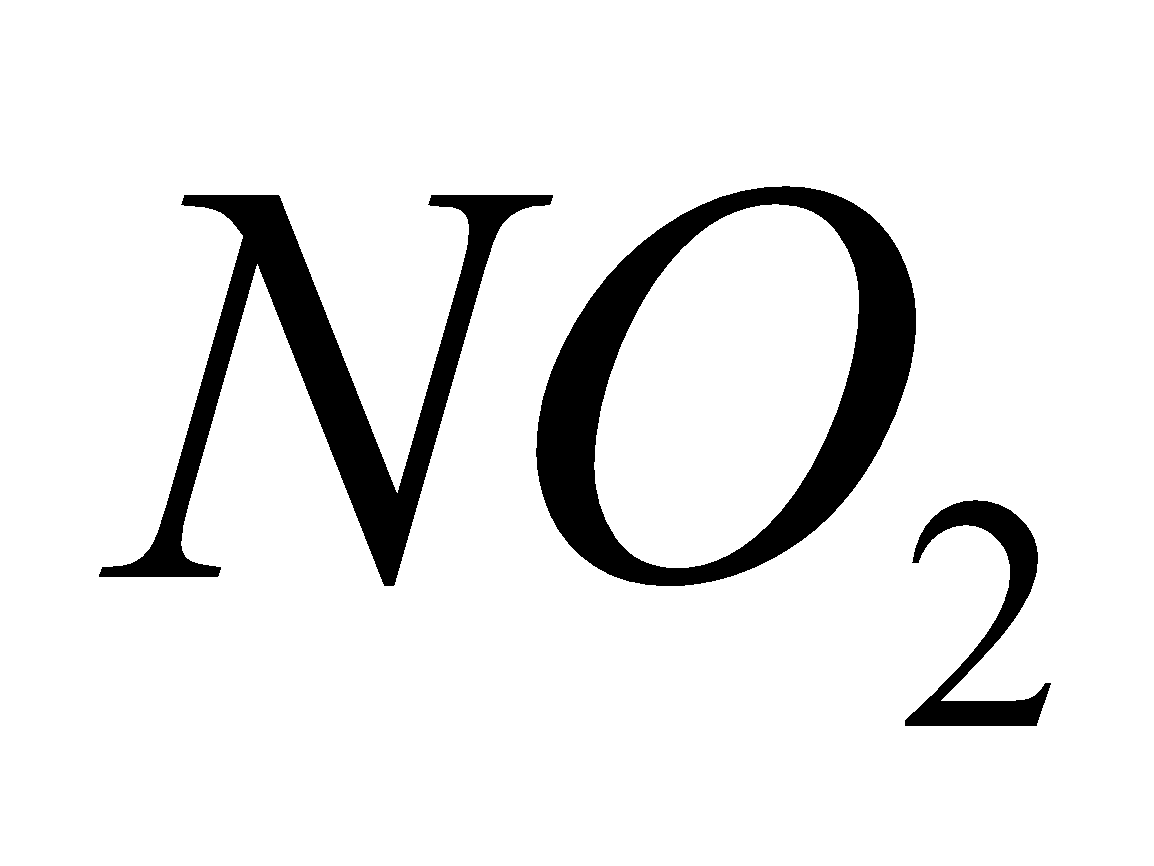 reacts with moisture to form acids.
reacts with moisture to form acids.
2 +
+
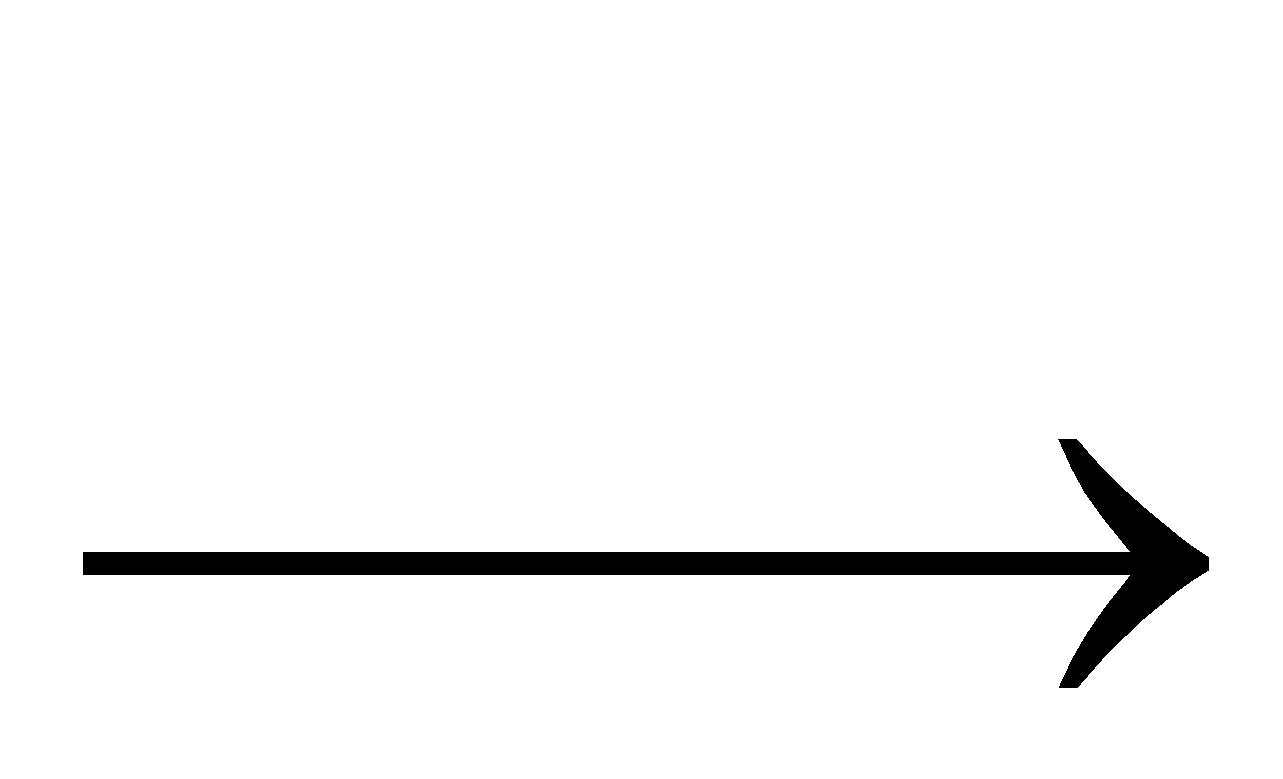
 +
+ 
3 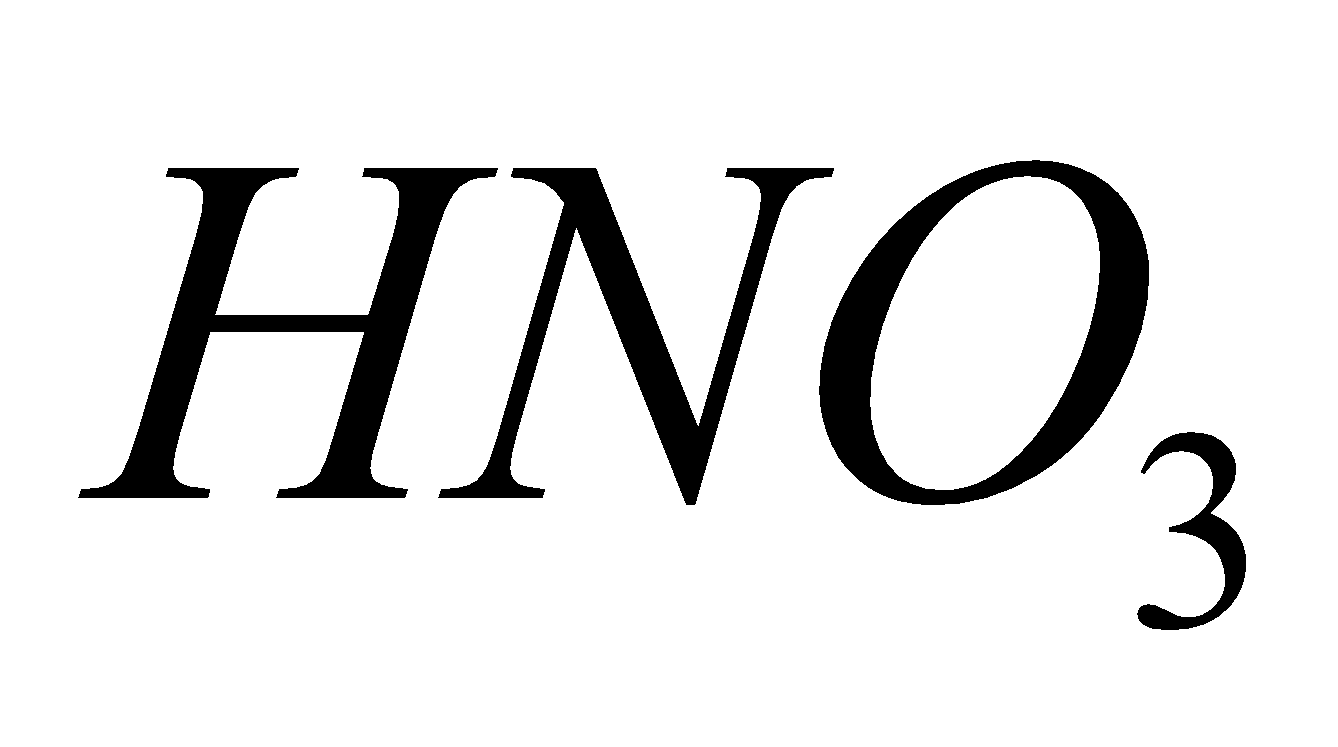
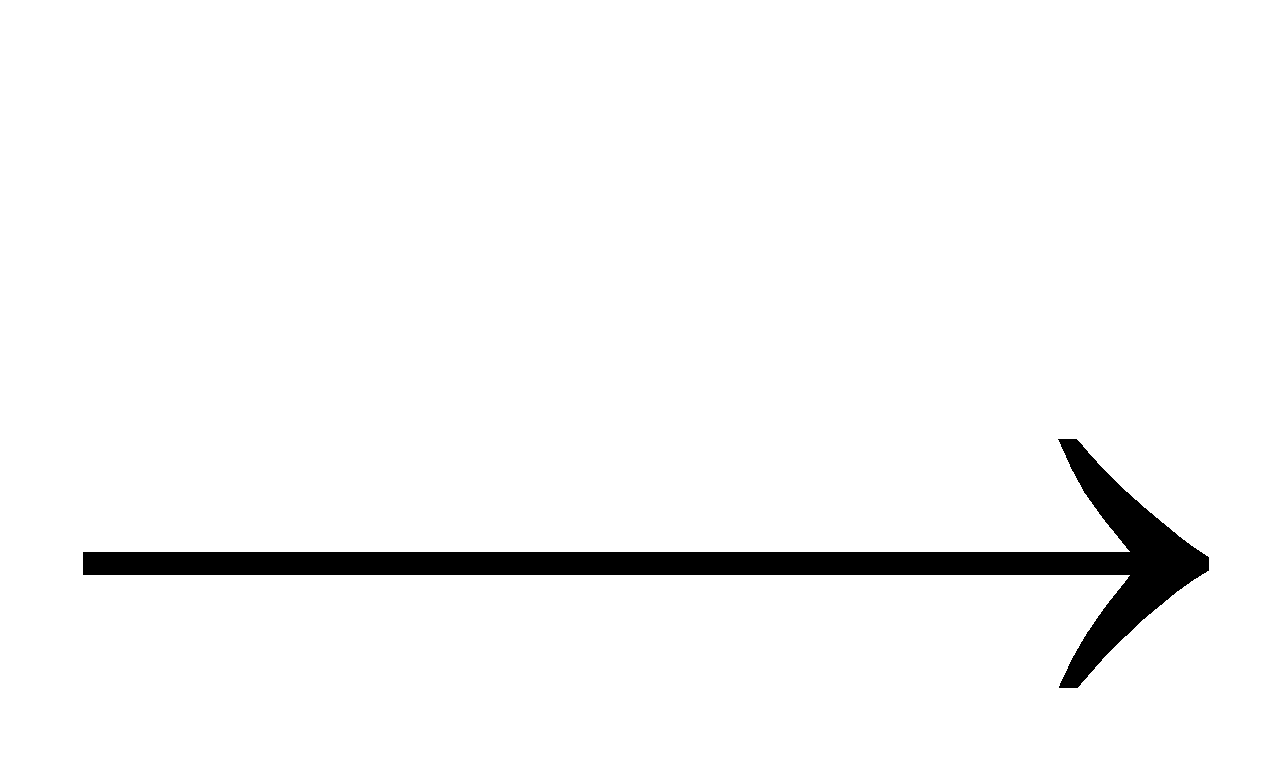 2 NO +
2 NO + 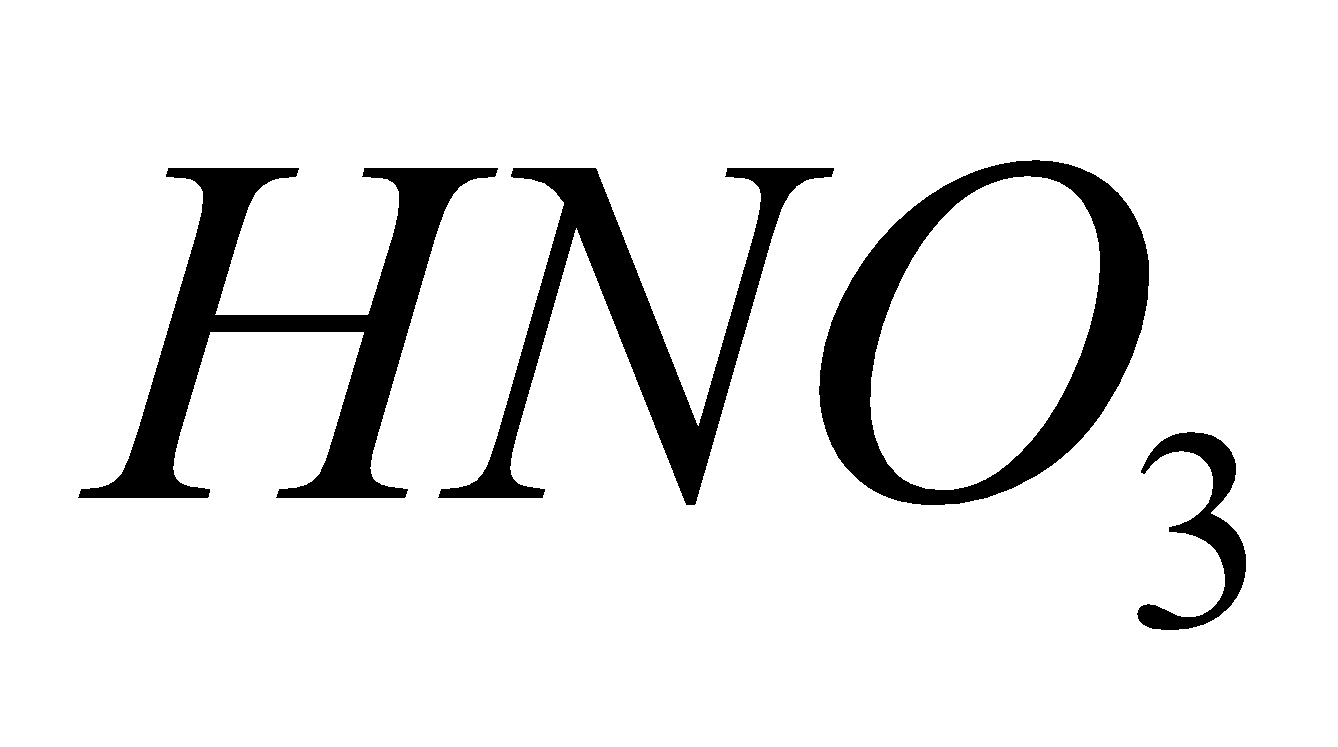 +
+ 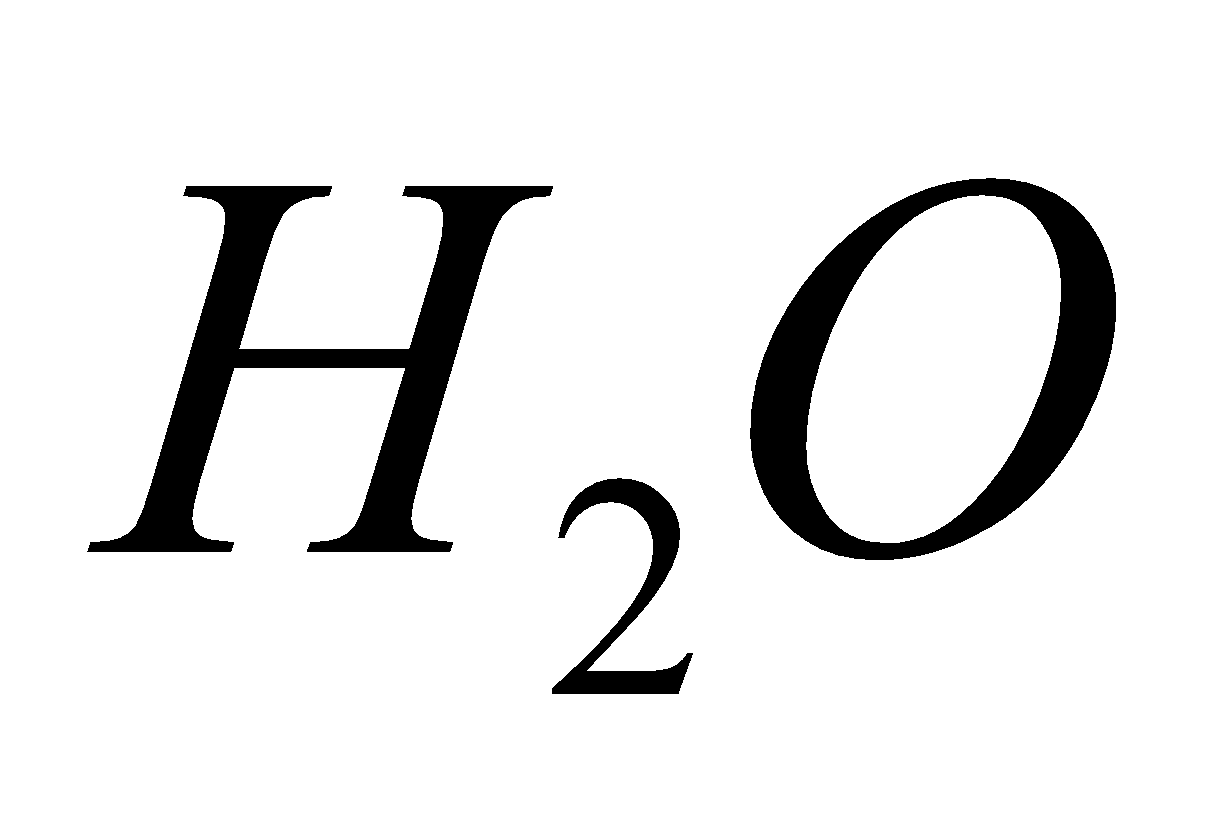
- Smoke, dust
Sources foundries, cement works, iron and steel works, gas works, power generating stations.
- Ammonia : Ammonia works
- Chlorine and hydrogen chloride : Chlorine works
- Chlorinated hydrocarbons : Dry cleaning works
- Mercaptans : Oil refineries, coke ovens
- Zn and Cd : Zinc industries
- Freon : Refrigeration works.
PHOTOCHEMICAL POLLUTANTS
The nitrogen dioxide by absorbing sunlight in blue and U.V. region decomposes into nitric oxide and atomic oxygen followed by a series of other reactions producing O3 formaldehyde, acrolein and peroxyacyl nitrates.
Ozone causes bronchial irritation even at 1 ppm level. Ozone affects tobacco plants, spinach, tomato, potato etc. The photochemical pollutants are powerful eye irritants. The reactions are as follows
RH + O 
 ,
,  +
+ 

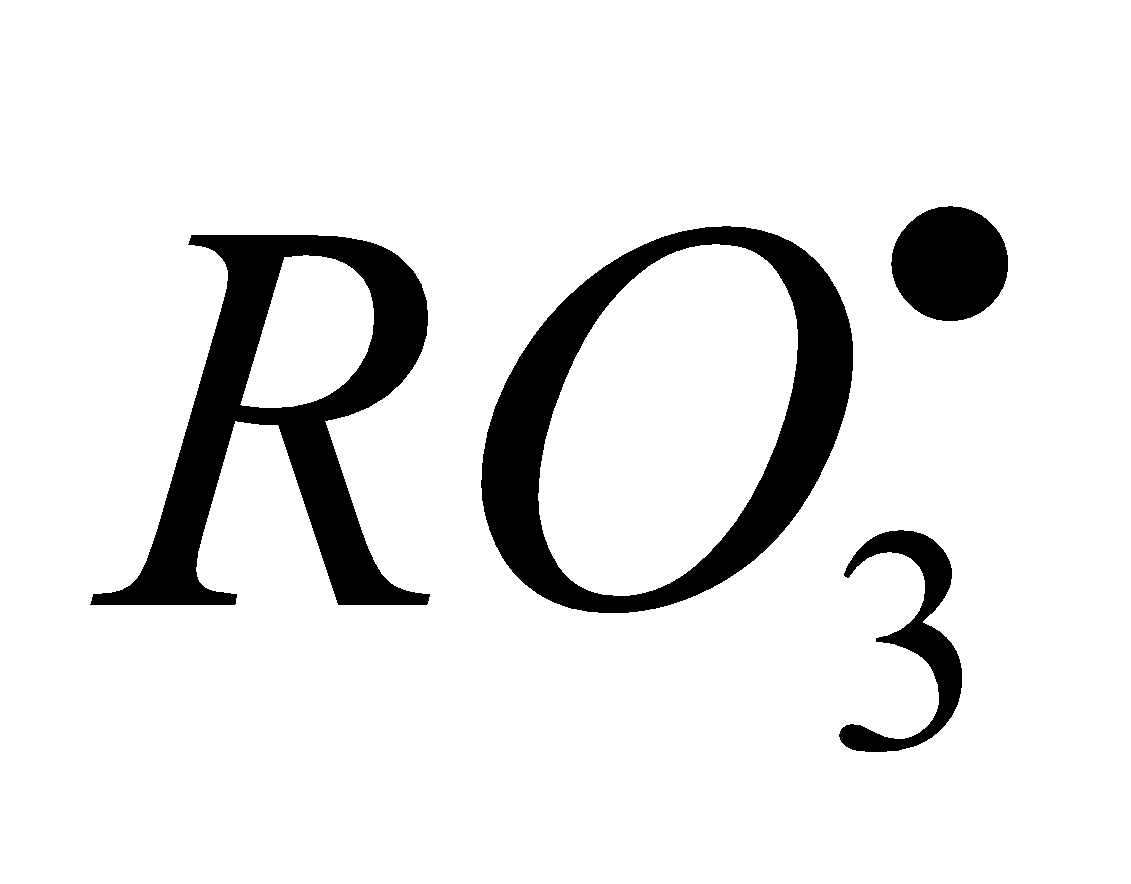
SMOG
It is a mixture of smoke (composed to tiny particles of carbon, ash and oil etc. from coal combustion) and fog in suspended droplet form. It is of two types :
London smog or classical smog : It is coal smoke plus fog. The fog part is mainly SO2 and SO3. It has sulphuric acid aerosol. It causes bronchial irritation and acid rain. It is reducing in nature.
Photochemical smog or Los Angeles smog : The oxidised hydrocarbons and ozone in presence of humidity cause photochemical smog.
Hydrocarbons + O2, NO2, NO, O, O3 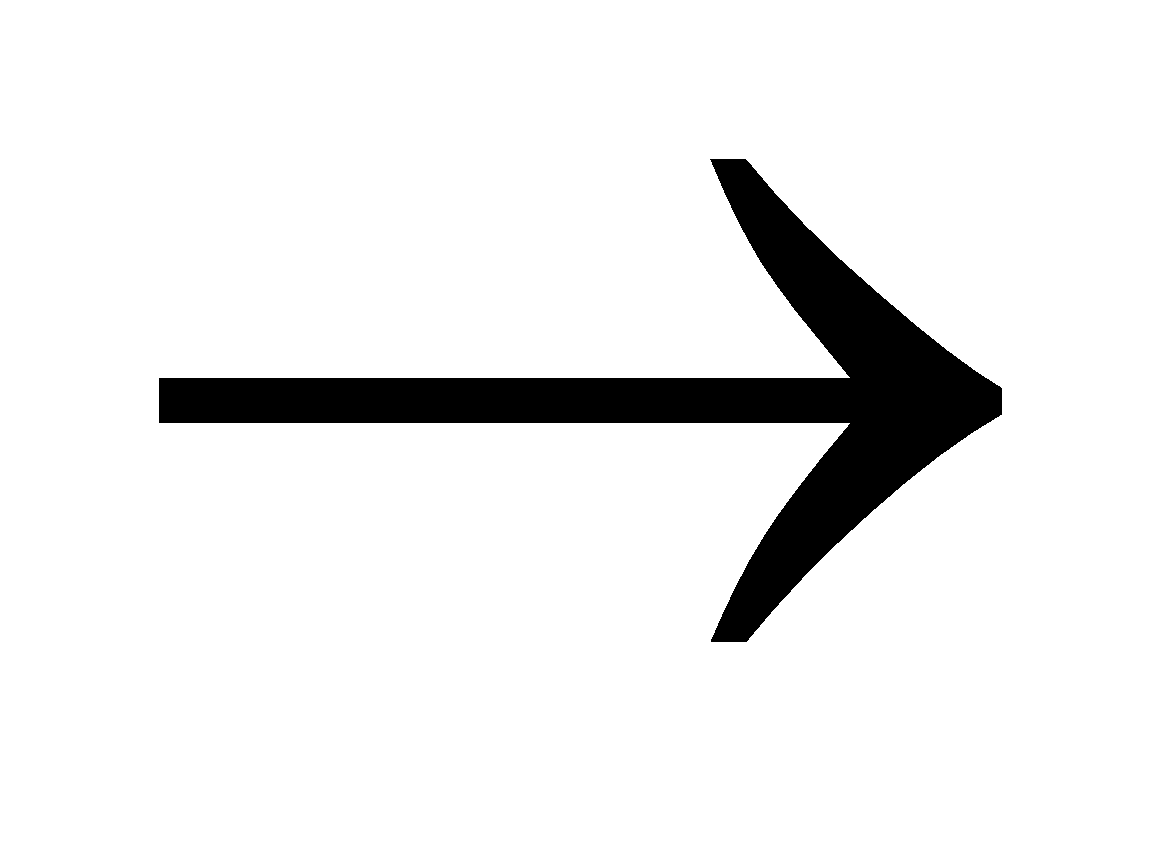 Peroxides, formaldehyde, peroxyacetyl nitrate (PAN), acrolein etc. It is oxidising in nature and causes irritation to eyes, lungs, nose, asthmatic attack and damage plants.
Peroxides, formaldehyde, peroxyacetyl nitrate (PAN), acrolein etc. It is oxidising in nature and causes irritation to eyes, lungs, nose, asthmatic attack and damage plants.
ACID RAIN
The oxides of C, N and S present in the atmosphere, dissolve in water and produce acids and lower the pH of water to below 5.5.
2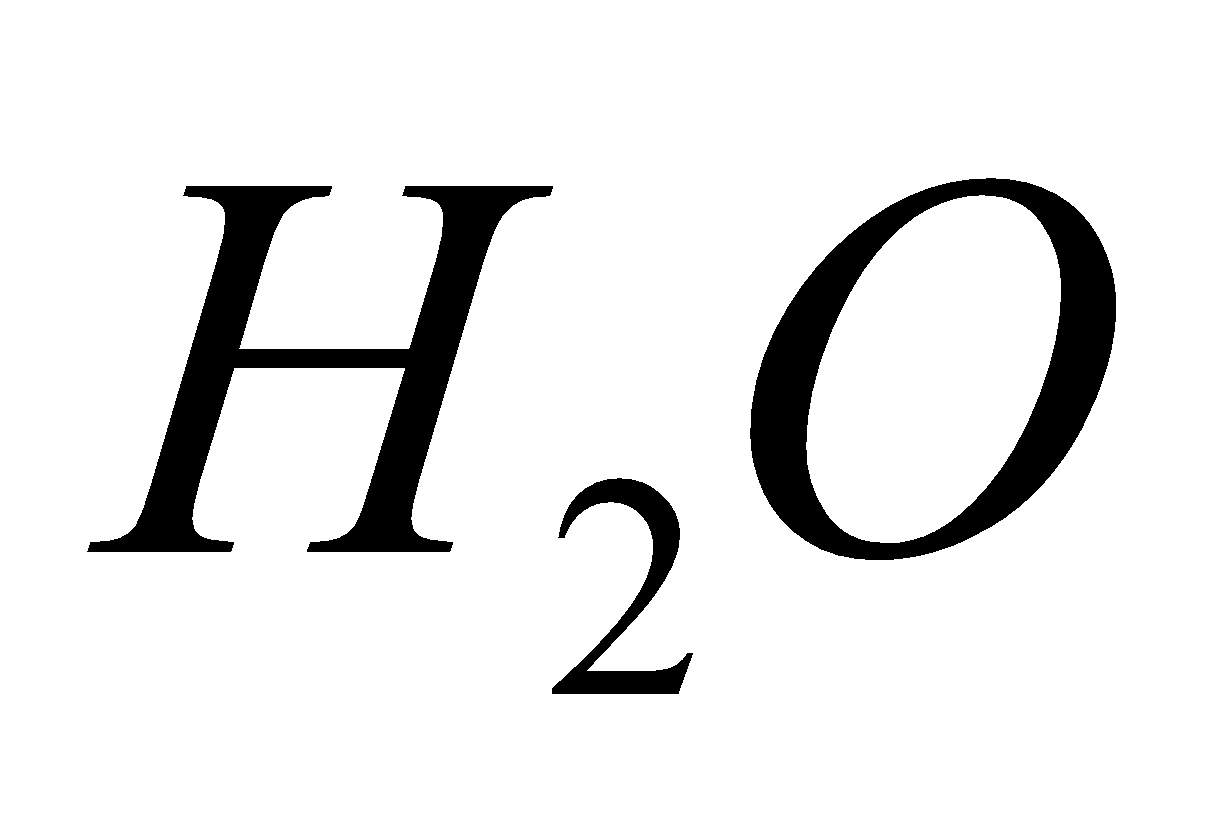 + 2
+ 2 +
+ 
 2
2
 2
2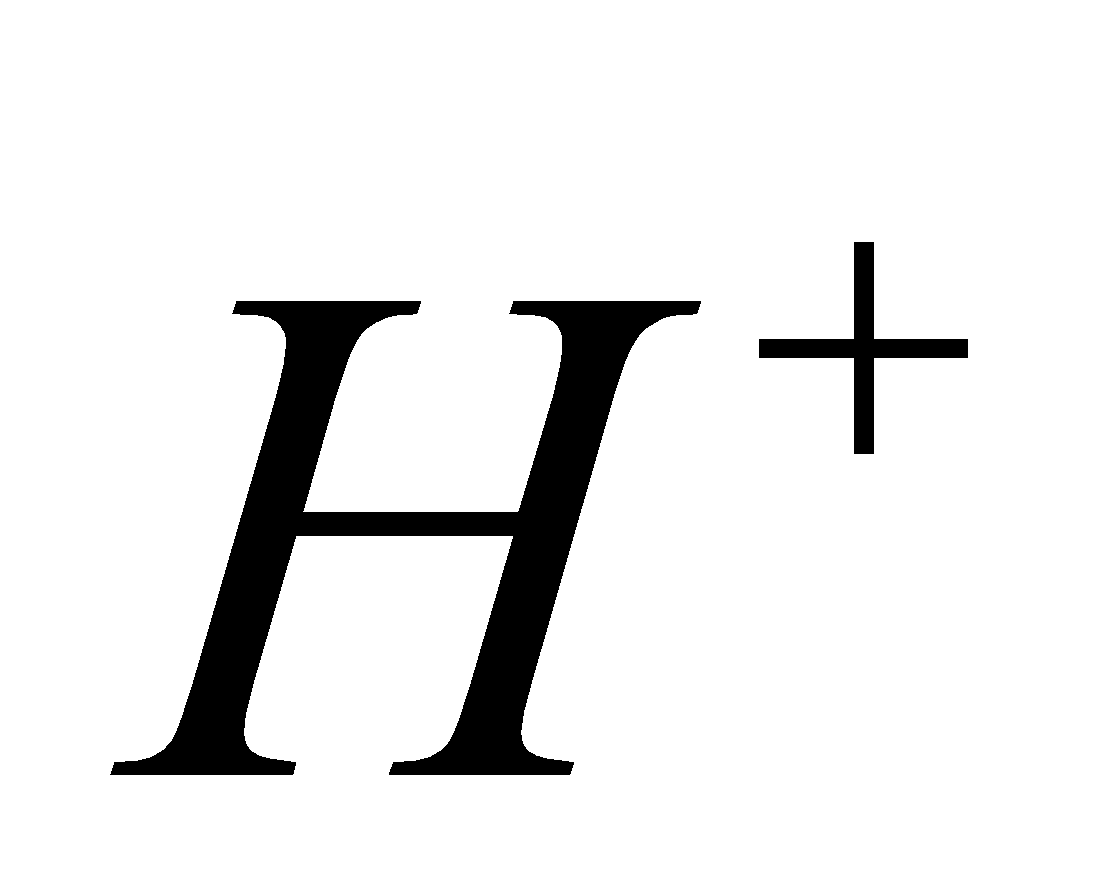 +
+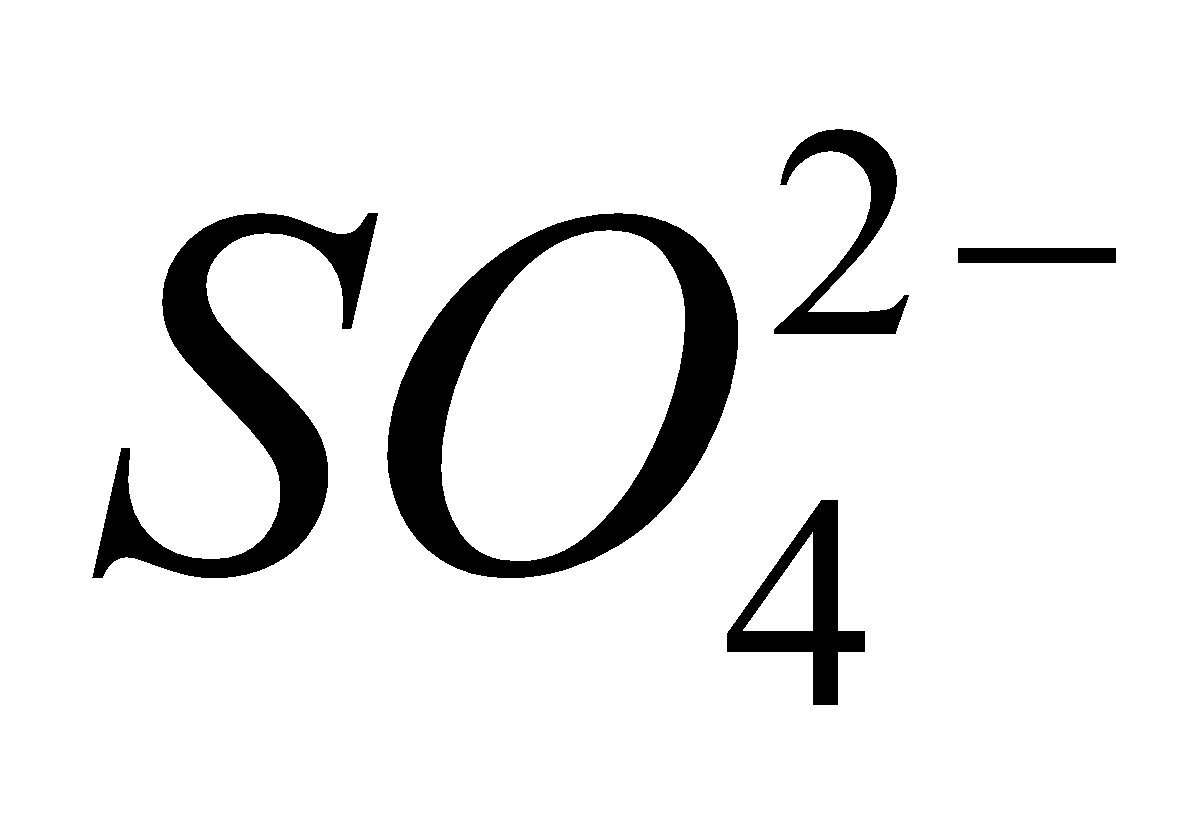
2 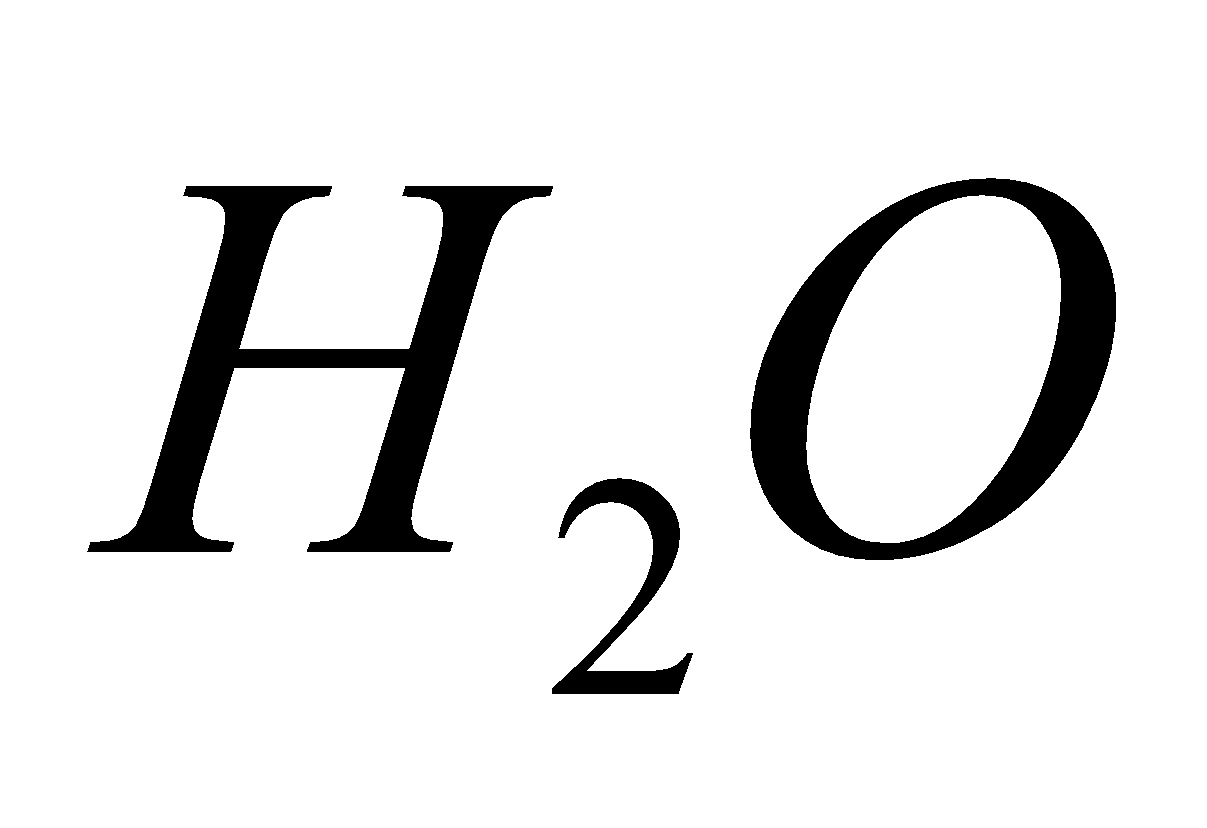 + 4
+ 4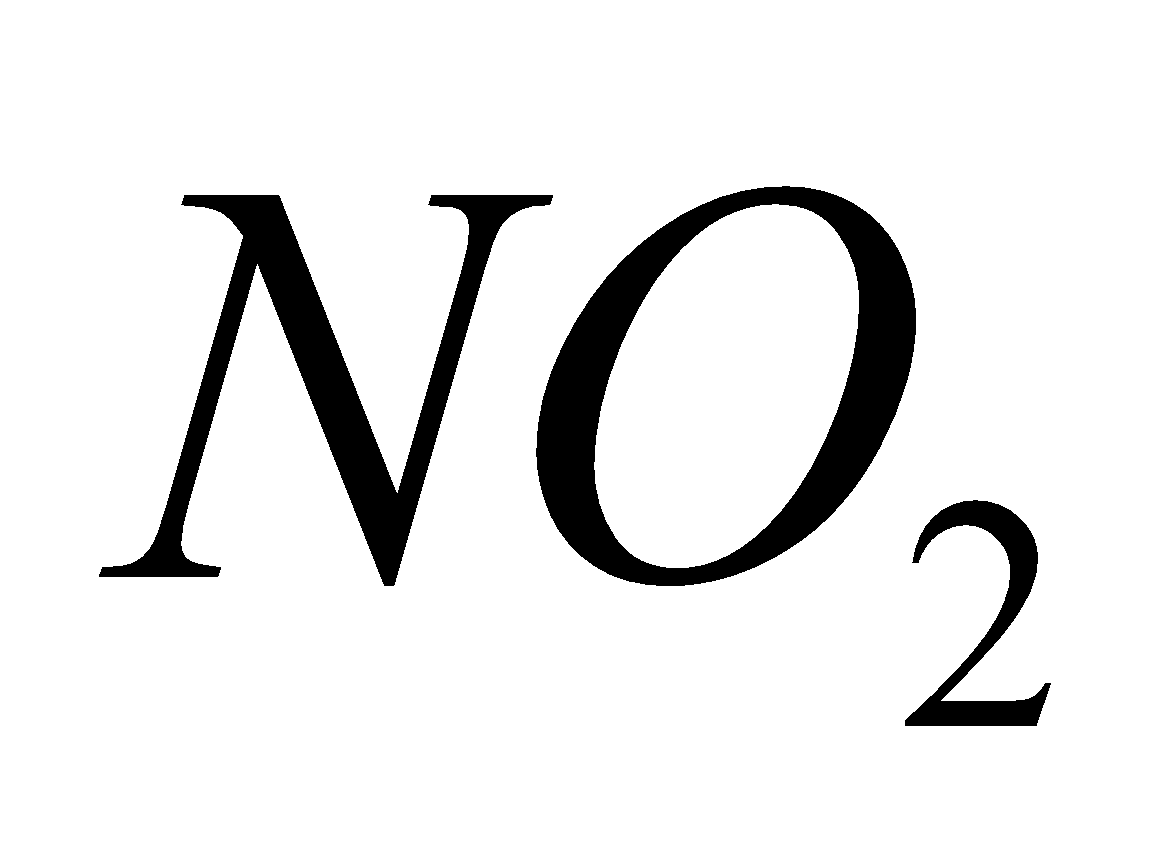 +
+ 
 4
4 
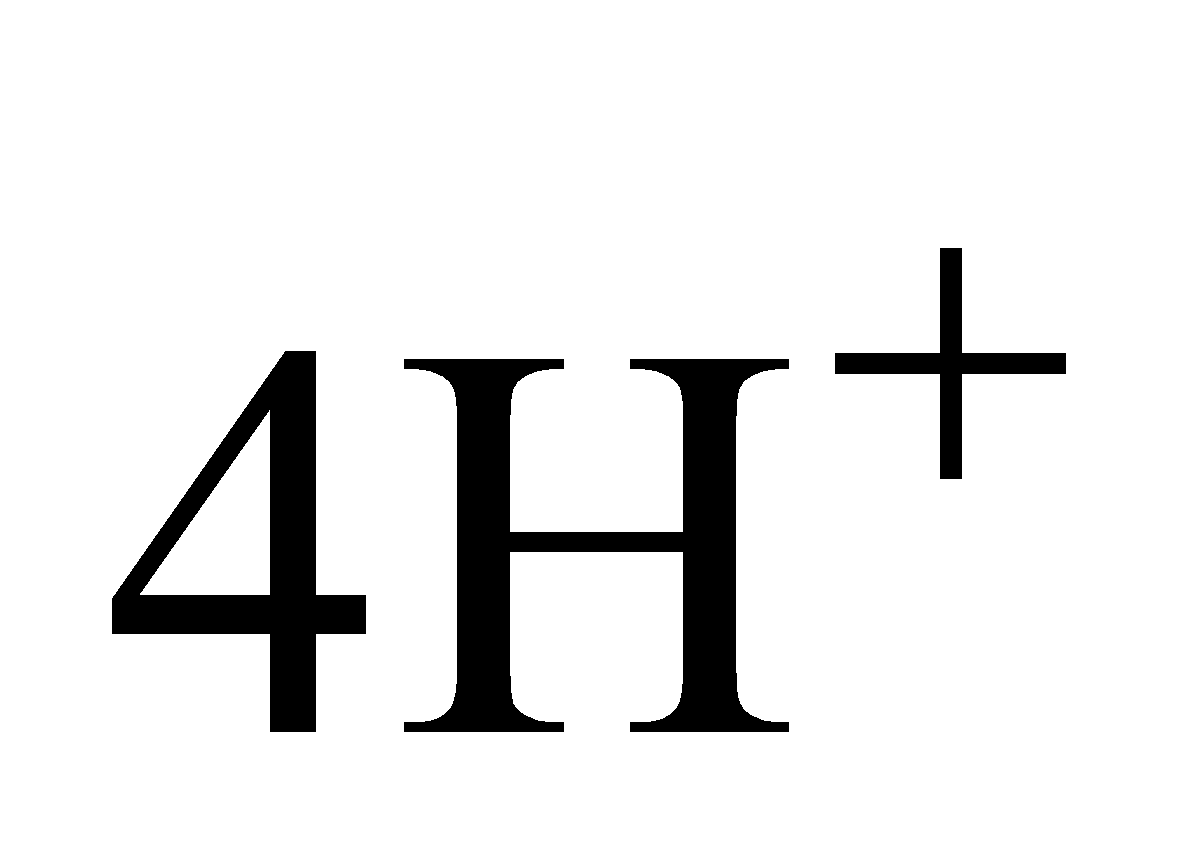 +
+ 
The acids are toxic to vegetation, react with marble and damage buildings.
Acids corrode water pipes and produce salts with heavy metals ions viz Cu, Pb, Hg and Al toxic in nature.
GREENHOUSE EFFECT
The retention of heat by the earth and atmosphere from the sun and its prevention to escape into the outer space is known as green house effect. Greenhouse gases such as , ozone, methane, the chlorofluorocarbon compounds and water vapour form a thick cover around the earth which prevents the IR rays emitted by the earth to escape. It gradually leads to increase in temperature of atmosphere.
Consequences of greenhouse effect
- Global warming would result in rise in sea level due to increased rate of melting of glaciers and floods.
- Increase in infectious diseases like malaria, dengue etc.
OZONE LAYER AND ITS DEPLETION
The ozone layer, existing between 20 to 35 km above the earth’s surface, shield the earth from the harmful U.V. radiations from the sun. The U.V. radiations cause skin cancer, cataract of eye, and harmful to vegetation.
Depletion of ozone is caused by oxides of nitrogen
reactive nitric oxide
NO +
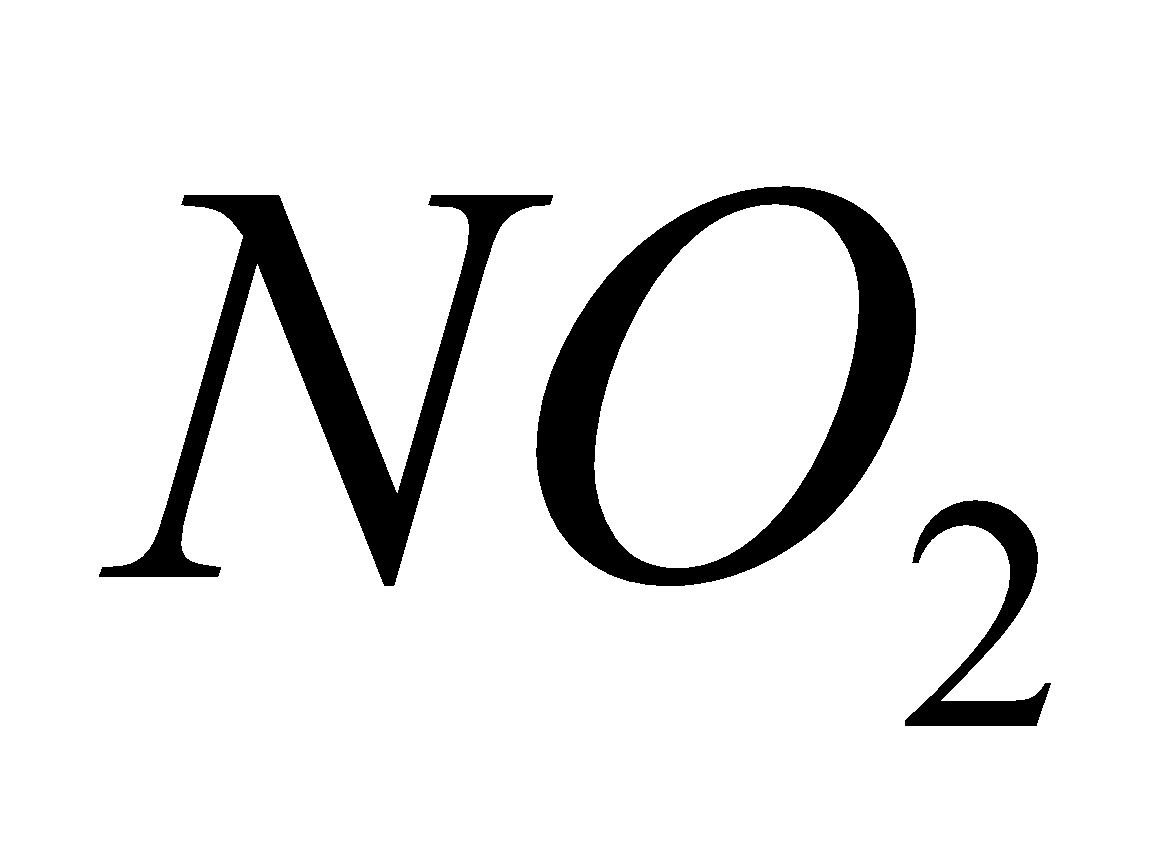 +
+ 
2O3 + h
 3O2 (Net reaction)
3O2 (Net reaction)
The presence of oxides of nitrogen increase the decomposition of 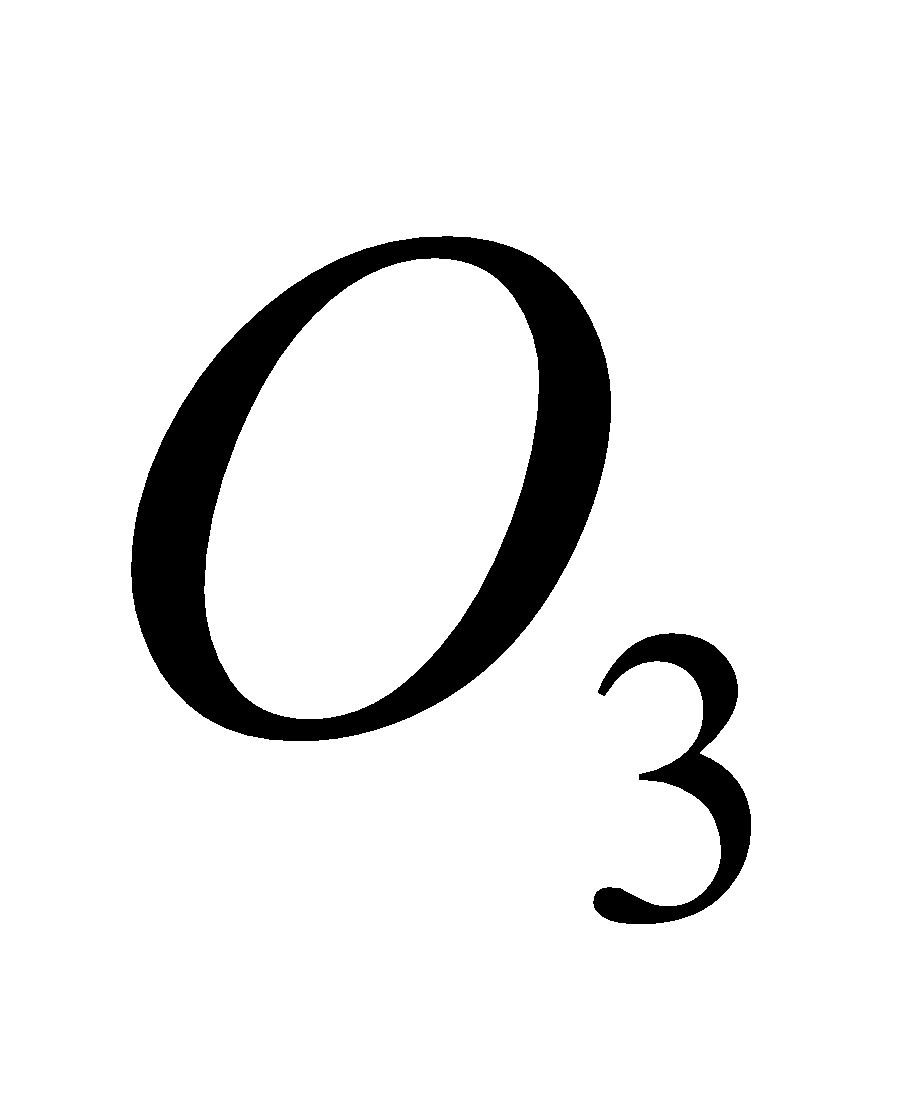 .
.
Depletion of ozone by chlorofluorocarbons.
CF2Cl2 + h
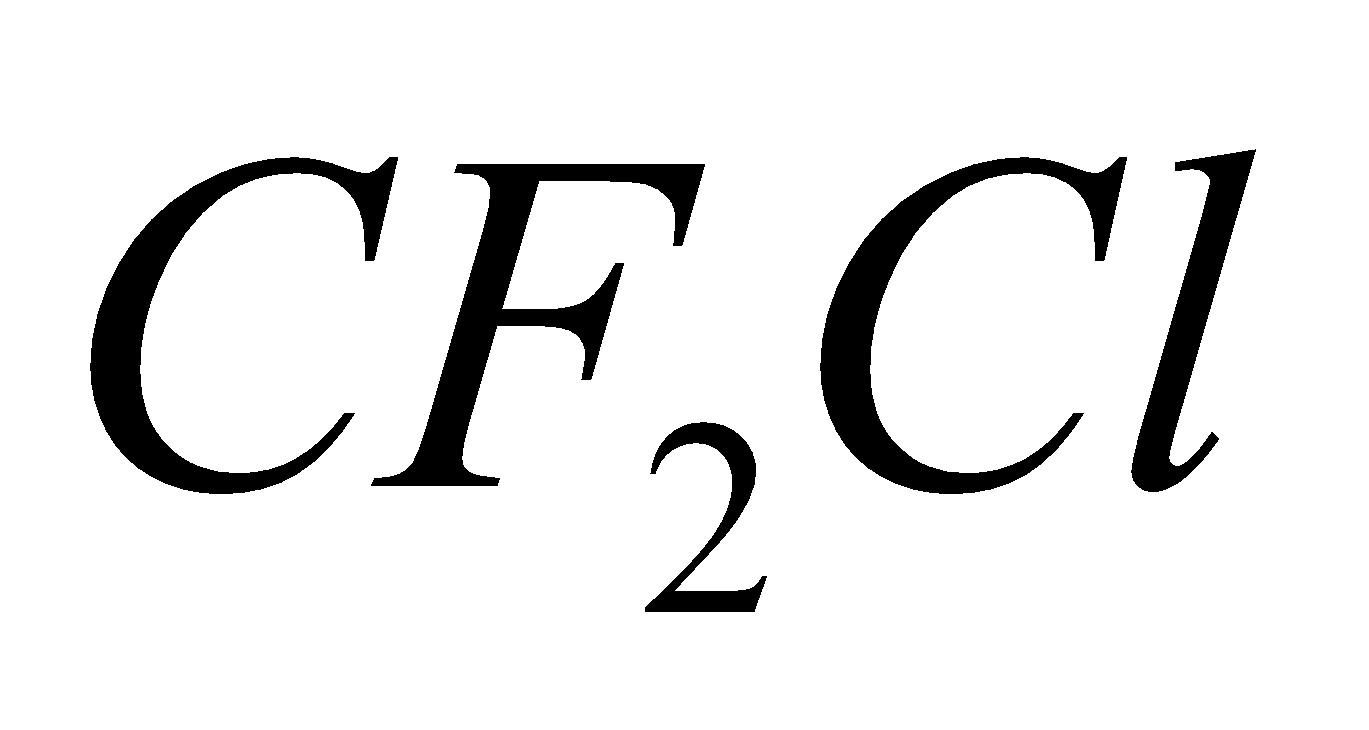 + Cl
+ Cl
Cl + 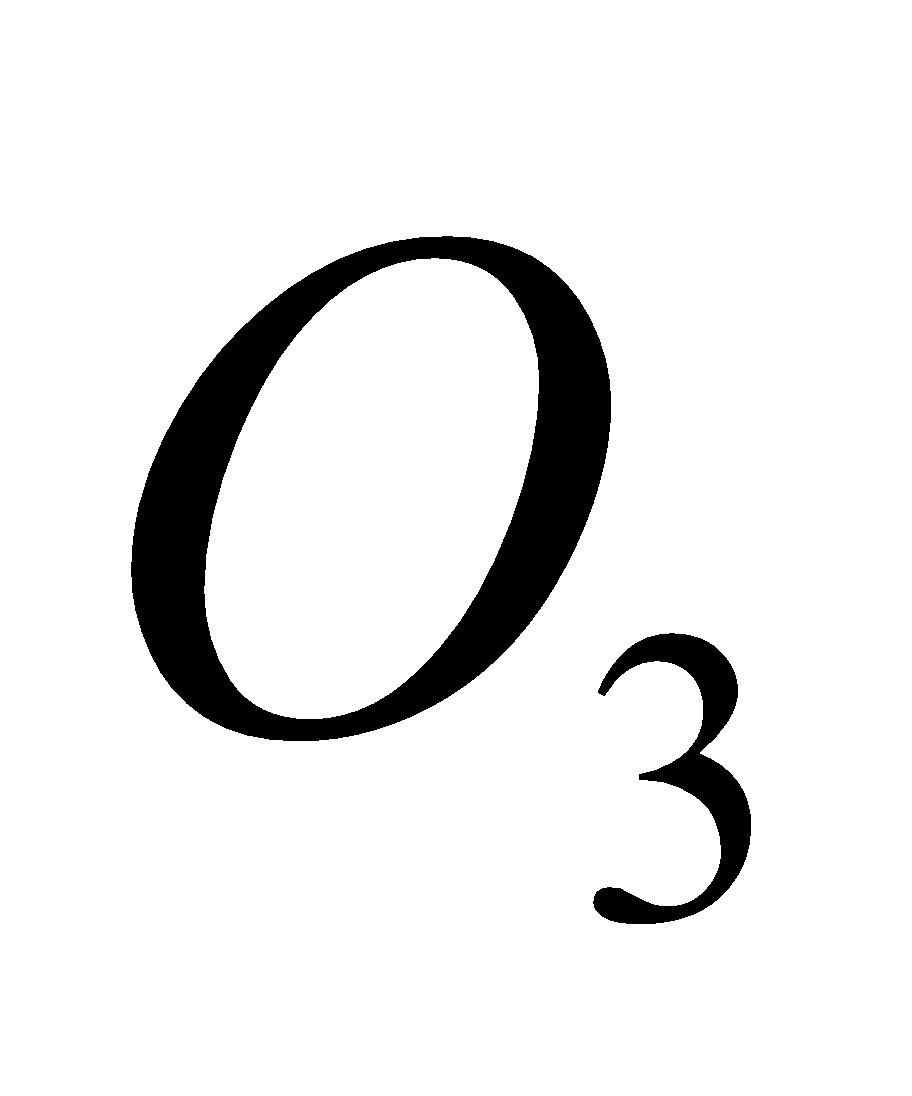
 ClO + O2
ClO + O2
ClO + O  Cl + O2
Cl + O2
CONTROL OF AIR POLLUTION
- Dissolving HCl, HF, SiF4 in water and SO2, Cl2, H2S in alkaline solution
- Adsorbing gas and liquids molecules over activated charcoal and silica gel
- Chemical reactions
2CuO +  + 2 S
+ 2 S 
 2
2 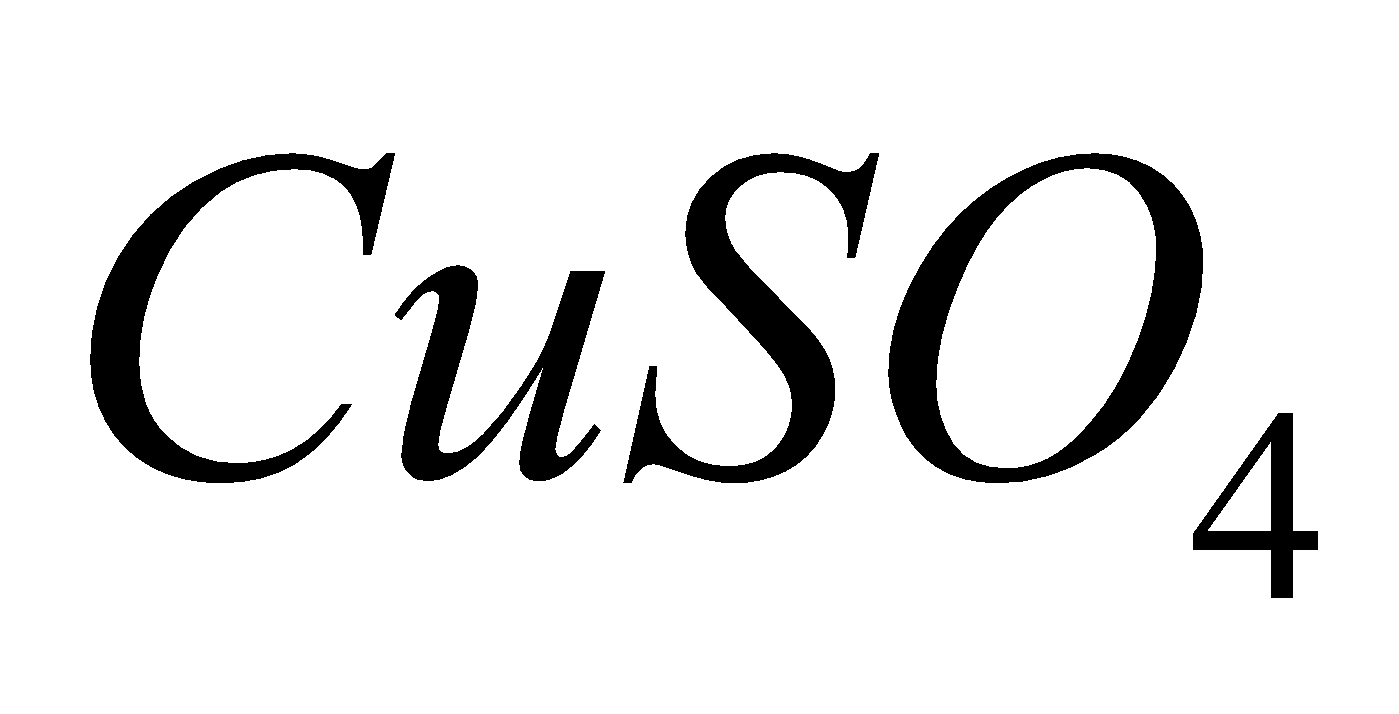
2 +
+  + 2
+ 2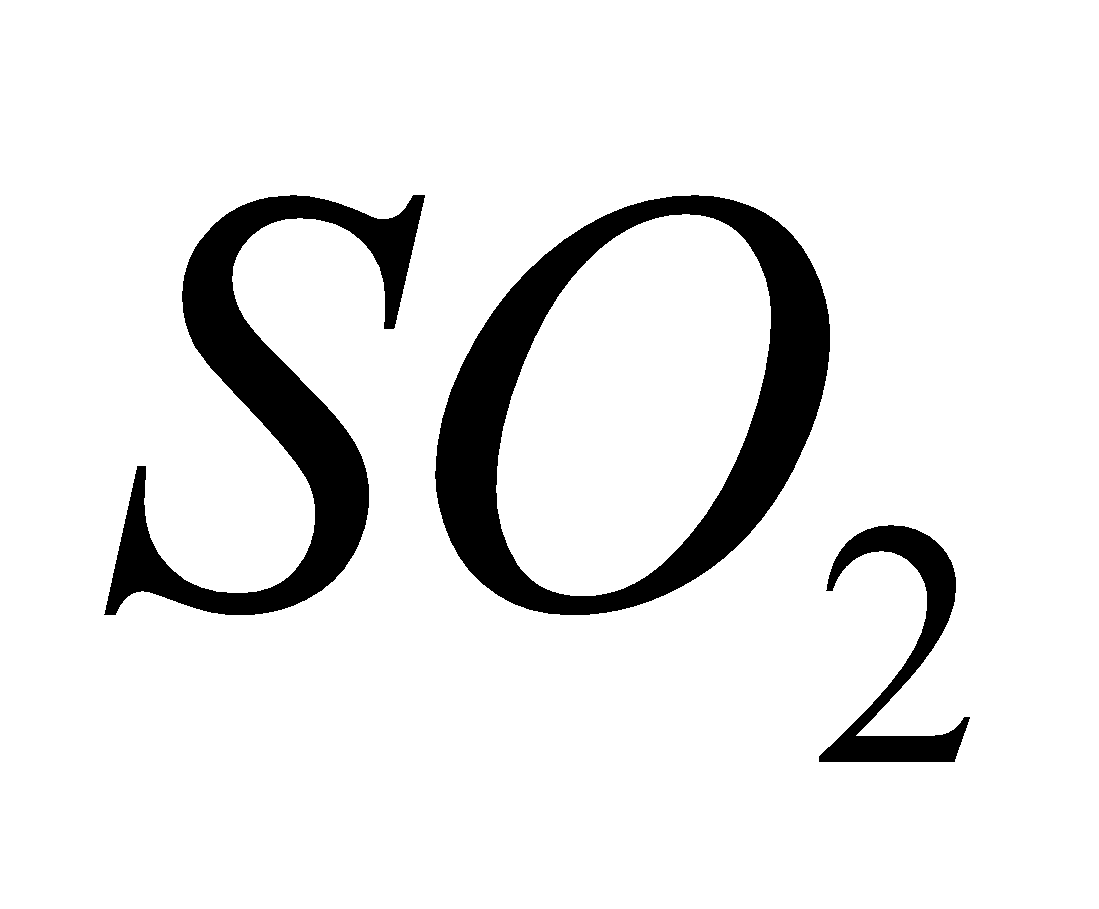
 2
2 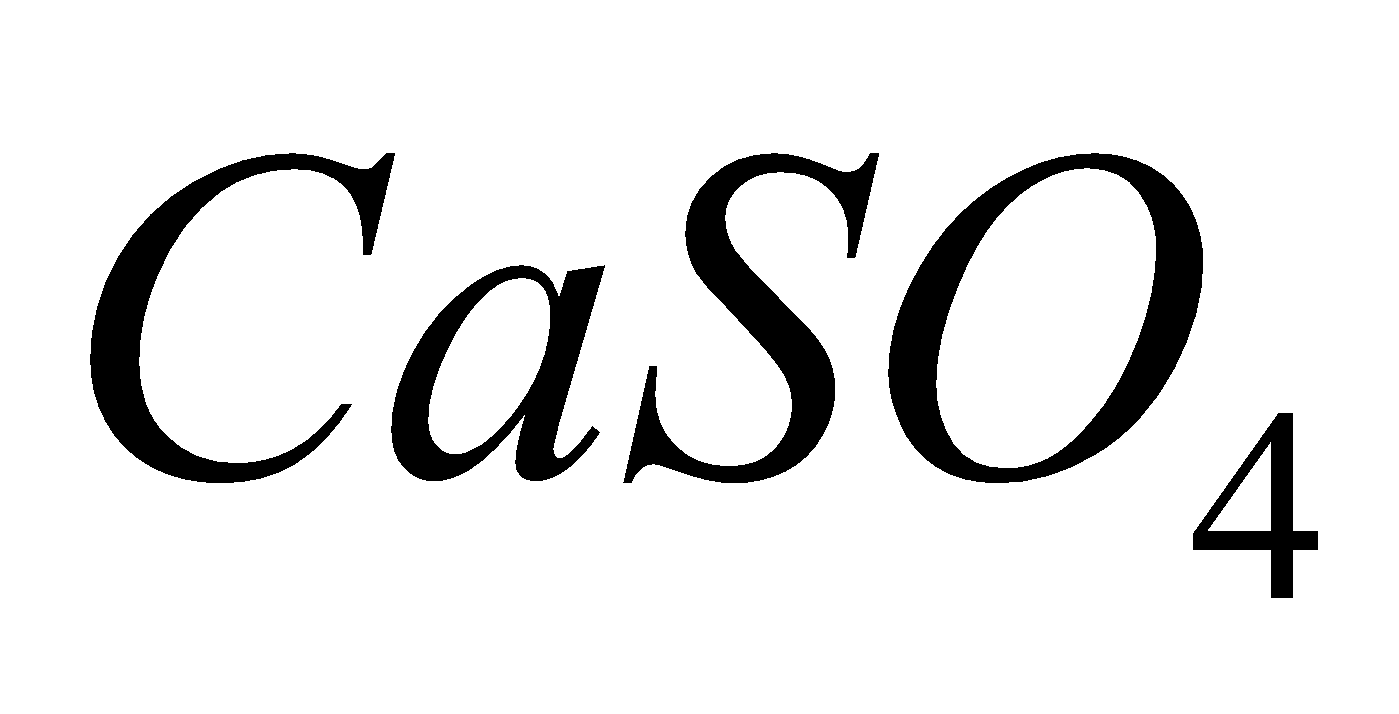 + 2
+ 2 
4 NO + 4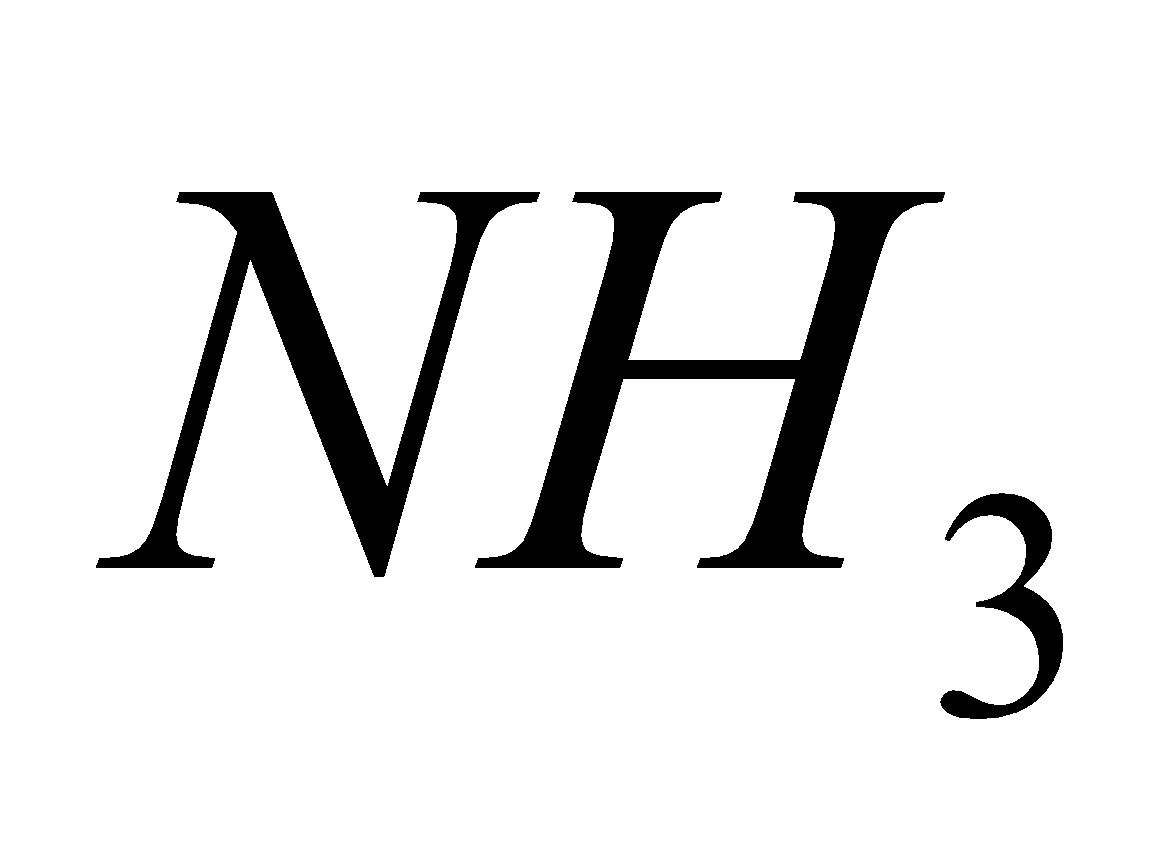 +
+ 
 2
2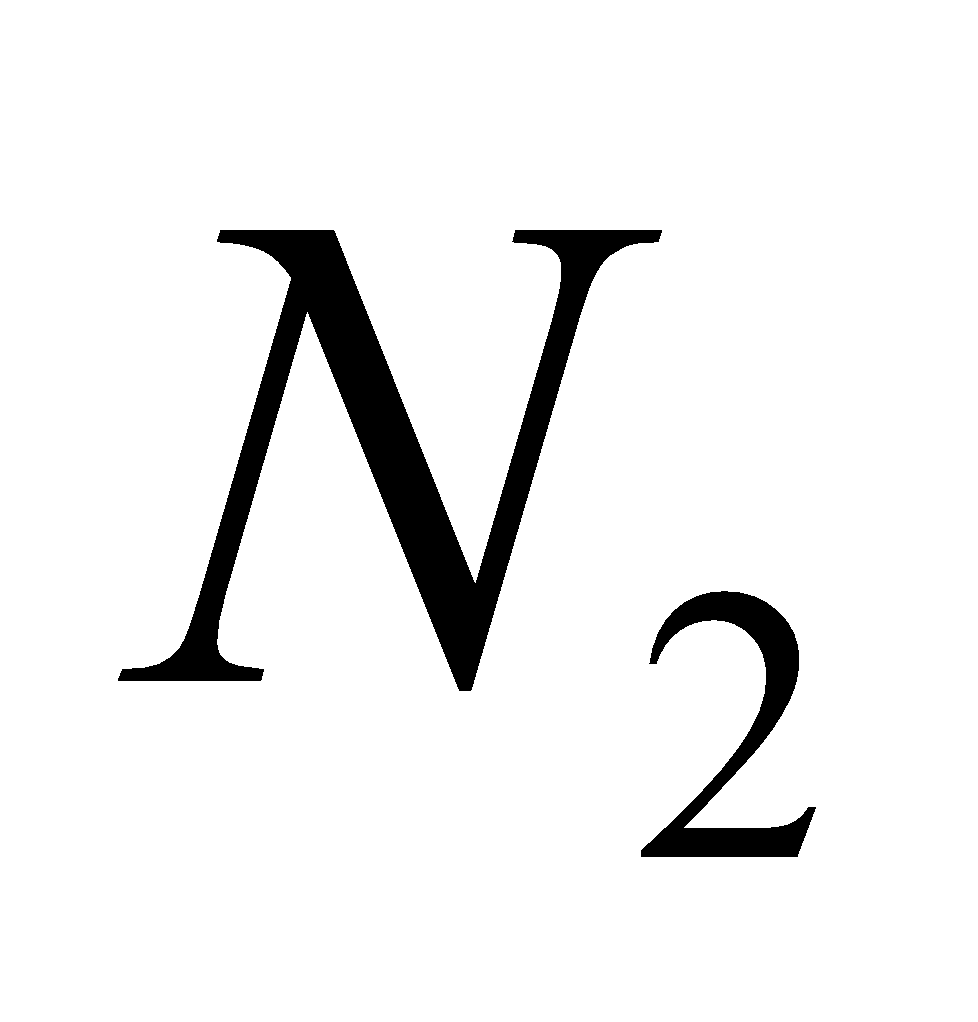 + 6
+ 6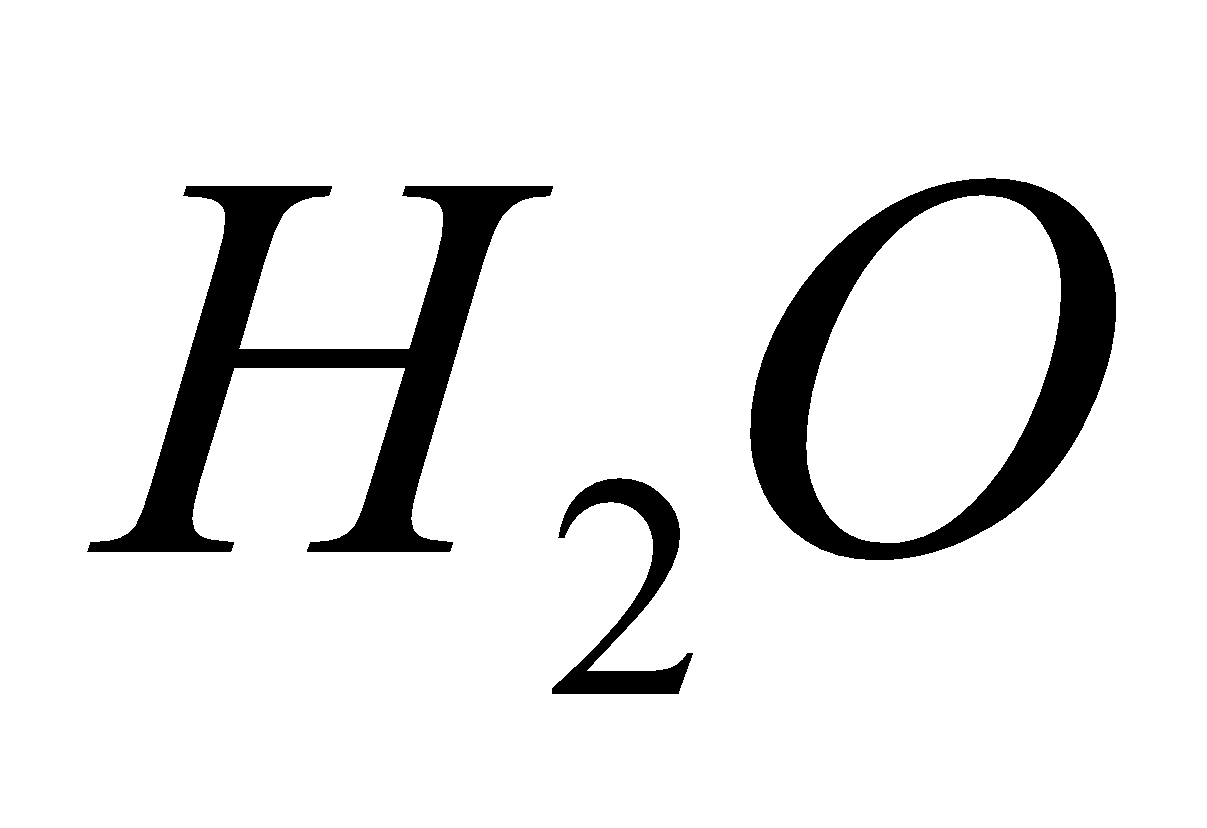
- Use of precipitators to settle charge particles
- Use of settling chambers under the action of gravity
- Use of natural gas in place of diesel, petrol etc.
WATER POLLUTION
The contamination of water by foreign substances which would constitute a health hazard and make it harmful for all purposes (domestic, industrial or agriculture etc.) is known as water pollution. The polluted water may have offensive odour, bad taste, unpleasant colour, murky oily etc.
SOURCES OF WATER POLLUTION
- Domestic sewage : Discharges from kitchens, baths, lavatories etc.
- Industrial waters : Wastes from manufacturing processes which includes acids, alkalies, pesticides, insecticides, metals like copper, Zinc, lead, mercury, fungicides etc.
- Oil : from oil spills or washings of automobiles
- Atomic explosion and processing of radioactive materials
- Suspended particles (organic or inorganic) viruses, bacterials algae protozoa etc.
- Wastes from fertilizer plants such as phosphates, nitrates ammonia etc.
- Clay : Ores, minerals, fine particles of soil.
EFFECTS OF IMPURITIES IN WATER
DISSOLVED SUBSTANCES
- Hardness : Corrosive effect on boils, alkalinity, laxative effect
- Fluorides : Mottling of teeth enamel, nervous and skeletal disorders, above 1 mg/litre causes fluorosis.
- Sulphates : Sulphates of Na, K, Mg cause diarrhoea
- Sodium chloride : It imparts bad taste to water
- Iron and manganese : Stain fabrics, bad taste, modify colours
- Lead : It damages kidney, liver, brain and central nervous system
- Cadmium and Mercury : Cause kidney damage
- Zinc : Causes vomiting, dizziness and diarrhoea
- Arsenic can cause cramps, paralysis and death
- Phosphates from fertilizers : They promote algae growth and reduce D.O. concentration of water. This process is known as eutrophication.
- Anionic detergents (eg. alkyl benzene sulphonates, ABS) : They produce stable foam, stabilise colloidal impurities and inhibit oxidation of organic compounds like phenol. ABS is not biodegradable.
- Hydrogen sulphide : Acidic, rotten-egg odour and corrosive to metals.
- Polychlorinated biphenyls : They are resistant to oxidation and cause skin disorders and are carcinogenic.
- Acid polluted water (pH < 3) : H2SO4 produced by oxidation of Iron pyrites (FeS2) harmful to life.
SUSPENDED IMPURITIES
- Parasitic worms : They cause infections
- Bacterias : Cause dysentery, typhoid, cholera
- Viruses : Cause enteroviral infections
- Algae : Cause foul odour, taste, turbidity
INTERNATIONAL STANDARDS FOR DRINKING WATER
Characteristics (mg/l)
|
Acceptable limit
|
Rejection limit
|
pH value
|
7-8.5
|
6.5-9.5
|
Total dissolved solids
|
500
|
1500
|
Total hardness (as CaCO3)
|
200
|
600
|
Fluorides
|
1.0
|
1.5
|
Chlorides
|
200
|
1000
|
Sulphates
|
200
|
400
|
Nitrates
|
45
|
45
|
Magnesium
|
30
|
150
|
Calcium
|
75
|
200
|
Zinc
|
5.0
|
15.0
|
Anionic detergents
|
0.2
|
1.0
|
Iron
|
0.1
|
1.0
|
Manganese / Copper
|
0.05
|
0.5
|
Phenolic compounds
|
0.001
|
0.002
|
Toxic Materials
eg. As, Cd, Cr, CN–, Pb, Se |
0.05-0.001
|
0.05-0.001
|
AEROBIC AND ANAEROBIC OXIDATION
The oxidation of organic compounds present in sewage in presence of good amount of dissolved or free oxygen (approx. 8.5 ml/l) by aerobic bacterias is called aerobic oxidation. When dissolved or free oxygen is below a certain value the sewage is called stale anaerobic bacterias bring out purification producing H2S, NH3, CH4, (NH4)2S etc. This type of oxidation is called anaerobic oxidation.
The optimum value of D.O. for good quality of water is 4-6 ppm (4-6 mg/l). The lower the concentration of D.O., the more polluted is the water.
BIOLOGICAL OXYGEN DEMAND (BOD)
It is defined as the amount of free oxygen required for biological oxidation of the organic matter by aerobic conditions at 20°C for a period of five days. Its unit is mg/l or ppm. An average sewage has BOD of 100 to 150 mg/l.
CHEMICAL OXYGEN DEMAND (COD)
It is a measure of all types of oxidisable impurities (biologically oxidisable and biologically inert organic matter such as cellulose) present in the sewage. COD values are higher than BOD values.
COD DETERMINATION
A known volume of sample is refluxed with known volume of standard K2Cr2O7 + dil. H2SO4 in presence of Ag2SO4 (catalyst) for 1½ hours. The unreacted K2Cr2O7 is then titrated against FeSO4.(NH4)2SO4.6H2O solution. The oxygen equivalent of K2Cr2O7 consumed is taken as a measure of COD.
1 ml. of 1 N K2Cr2O7 0.008 g oxygen
0.008 g oxygen
CONTROL OF WATER POLLUTION
The water pollution can be reduced by following techniques
- Recycling of waste water : by aeration and use of trickling filter
- Use of chemicals : Effective filtration and chlorination
- Special techniques : Such as adsorption, ion-exchangers, reverse osmosis, electrodialysis etc.
- Waste-water reclamation : Sewage water can be directly used for irrigation and fish farms. Since it contains N, P and K.
SOIL OR LAND POLLUTION
The addition of substances in an indefinite proportion changing the productivity of the soil is known as soil or land pollution.
SOURCES OF SOIL POLLUTION
- Agricultural pollutants : Chemicals like pesticides, fertilizers, fumigants, insecticides, herbicides, fungicides.
- Domestic refuge and industrial wastes
- Radioactive wastes from research centres, and hospitals
- Soil conditioners containing toxic metals like Hg, Pb, As, Cd etc.
- Farm wastes from poultries, dairies and piggery forms
- Improper disposal of human and animal excreta.
- Pollutants present in air from chemical works
SEWAGE TREATMENT
The artificial treatment is called sewerage and involves the following steps :
- Preliminary process : Passing sewage through screens to remove large suspended matter and then through mesh screens to remove solids, gravels, silt etc.
- Settling process (sedimentation) : The residual water when allowed to stand in tanks, the oils and grease float on the surface and skimmed off and solids settle down. The colloidal material is removed by adding alum, ferrous sulphate etc. and we get primary sludge.
- Secondary treatment or Biological treatment : It is aerobic chemical oxidation or aeration which converts carbon of the organic matter to CO2, nitrogen into NH3 and finally into nitrites and nitrates, dissolved bases form salts such as NH4NO2, NH4NO3 and Ca(NO3)2 etc. and secondary sludge is obtained.
The primary and secondary sludge (combined) is subjected to anaerobic digestion to CO2 and CH4
- Tertiary treatment : It is treatment of waste water with lime for removal of phosphate which is coagulated by adding alum and ferric chloride and removed by filtration. Water is disinfected by adding chlorine.
Secondary sludge forms a good fertilizer for soil as it contains nitrogen and phosphorous compounds.
PESTICIDES
The chemical substances used to kill or stop the growth of unwanted organisms are called pesticides. They are further classified as
INSECTICIDES
They are used to kill insects. The most common insecticides are
- Aldrin
HERBICIDES
They are used to kill weeds e.g.
- Triazines
The (III) and (IV) are not used now a days.
FUNGICIDES
They are used to stop or kill fungus e.g.
RODENTICIDES
They are used to kill rodents e.g.










