PRINCIPLES RELATED TO PRACTICAL CHEMISTRY (PART-1)
DETECTION OF NITROGEN, SULPHUR AND HALOGENS IN ORGANIC COMPOUNDS
DETECTION OF NITROGEN
- Soda-lime test
organic compound + NaOH  smell of NH3
smell of NH3 
(Not reliable since –NH2, –NO2 and – N = N-groups do not respond)
- Lassaigne’s test
(Common for N, S and X (halogens)
Lassaigne’s filtrate or sodium extract is prepared by fusing the organic compound with Na in ignition tube. Fused mass is dissolved in water, boiled and filtered. The filtrate is sodium extract which contains
Na + C + N NaCN sodium cyanide
NaCN sodium cyanide
2Na + S Na2S Sodium sulphide
Na2S Sodium sulphide
Na + X  NaX (Sodium halide)
NaX (Sodium halide)
Na + C + N + S NaCNS (Sodium Sulphocyanide)
NaCNS (Sodium Sulphocyanide)
TEST FOR NITROGEN
Sod. extract + freshly prepared FeSO4 solution + FeCl3 solution + dil. H2SO4 green or blue colouration or sometimes blood red colour
green or blue colouration or sometimes blood red colour
2 NaCN + FeSO4  Fe(CN)2 + Na2SO4
Fe(CN)2 + Na2SO4
4 NaCN + Fe(CN)2 Na4 [Fe(CN)6]
Na4 [Fe(CN)6]
3 Na4[Fe(CN)6] + 4 FeCl3 Fe4[Fe(CN)6]3 + 12NaCl
Fe4[Fe(CN)6]3 + 12NaCl
ferric ferrocyanide
(prussian blue)
FeCl3 + 3 NaCNS  Fe(CNS)3 + 3NaCl
Fe(CNS)3 + 3NaCl
Ferric sulphocyanide
(Blood red)
(NH2.NH2 and diazo compounds do not give this test, diazo compounds are decomposed to give N2 and NH2.NH2 does not contain C)
TEST FOR SULPHUR
TEST FOR HALOGENS
Sod. extract + Conc. HNO3 + AgNO3 Solution
- If white precipitate soluble in NH4OH
Cl– present
- If light yellow precipitate sparingly soluble in NH4OH
Br– present
- If yellow precipitate insoluble in NH4OH
I– present
- Function of Conc. HNO3 : It decomposes NaCN and Na2S to avoid their interference
- Layer test for bromine and iodine
Sod. extract
- Beilstein's test
Organic compounds containing halogens when heated over Cu wire loop, give blue or green colour flame due to formation of volatile copper halides.
(Not reliable since thiourea, urea, pyridine and organic acids also give this test)
DETECTION OF THE FUNCTIONAL GROUPS
ALCOHOLIC GROUP (–OH)
- Ceric ammonium nitrate lest
Treat 2 drops of the organic substance with 0.5 c.c. of ceric ammonium nitrate solution and dilute with 2 c.c. of water. A red colour indicates alcoholic group
- Sodium test
2 cc of organic substance +a piece of anhydrous CaCl2 to absorb water if present. Transfer the organic substance to another test tube and add a dry piece of sodium. Effervescence indicate the presence of an alcohol
- Evolution of HCl
Take 0.5 cc or 0.3 g of the original substance in a dry test tube and add to it about 6 drops of acetyl chloride or PCl5. Vigorous reaction takes place with the evolution of HCl fumes
The same test could be repeated with acetic anhydride when a characteristic. Fruity odour is obtained
- Xanthate test
To 1 c.c. of the conc. aq solution of the original substance add 2 pallets of KOH. Heat and cool. Add 1 c.c. of the ether and 2-3 drops of CS2. Formation of yellow ppt. indicate the presence of alcoholic group
ROH + KOH  ROK + H2O
ROK + H2O
Distinction between 1º, 2º and 3º alcohol
2 ml of organic compound + 8 ml of Lucas reagent and shake.
Separation of an insoluble layer at once 3º alcohol Appearance of cloudiness within 4-5 minutes 2º alcohol solution remains clear 1º alcohol.
PHENOLIC GROUP (–OH)
- Ferric Chloride Test
2 c.c. of aqs. or alcoholic solution + 2 drops of ferric chloride deep colour change shows the presence of phenols.
deep colour change shows the presence of phenols.
- Liebermann's Reaction
1 c.c. of conc.  small amount of O.S. + few crystals of
small amount of O.S. + few crystals of pour the contents of the test tube to a beaker half full of water
pour the contents of the test tube to a beaker half full of water
- Phthalein Test
0.5g O.S. + 1.0 g phthalic anhydride + drop of conc. 
 add dil. NaOH solution
add dil. NaOH solution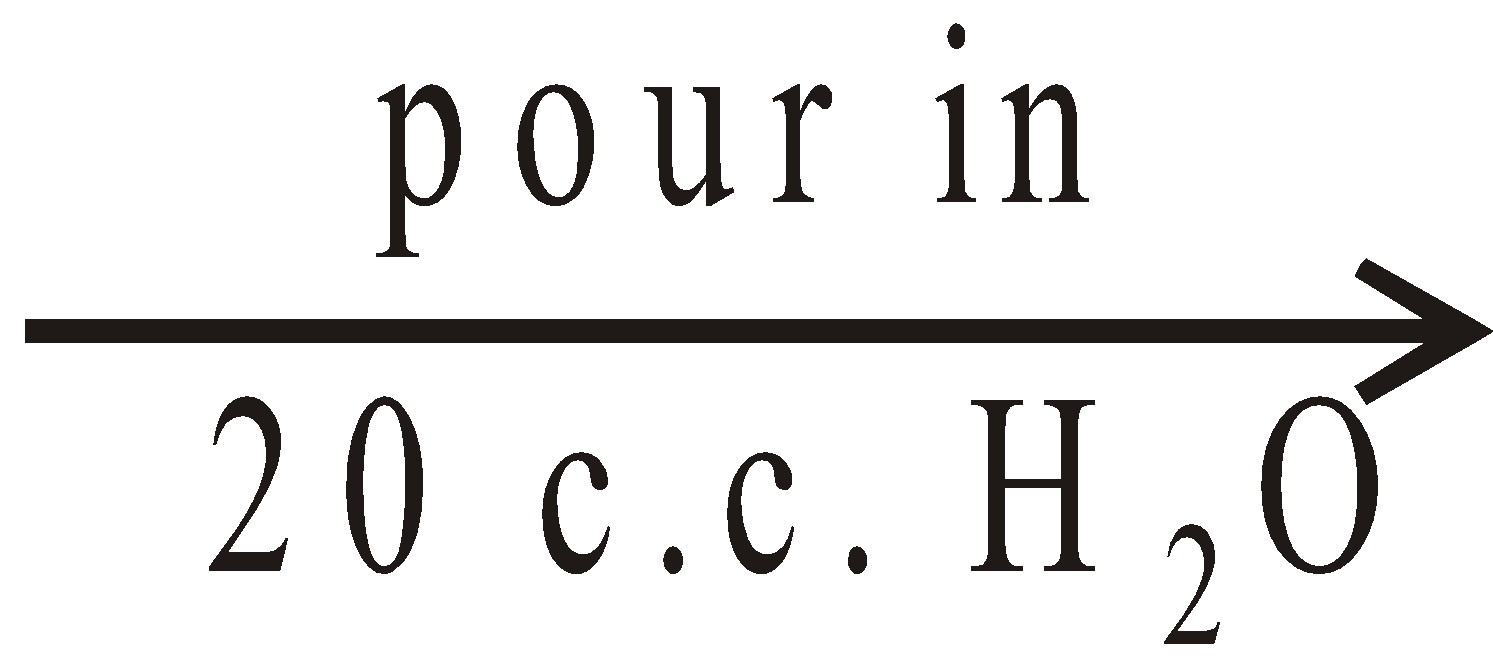 characteristic colour
characteristic colour
ALDEHYDIC GROUP (–CHO)
- Schiff’s reagent
5-6 drops or 0.1 g of O.S. + 2c.c. Schiff’s reagent
- Tollen’s reagent
5-6 drops or 0.1 g of O.S. + 2 c.c. Tollen’s reagent
- Fehling solution
5-6 drops or 0.1 g of O.S. + 5 c.c. Fehling solution
Note : Aromatic aldehydes do not respond to this test.
- Benedict’s solution
0.1 g of O.S. + 2-3 c.c. of Benedict’s solution
KETONIC GROUP 
- 2, 4–Dinitrophenylhydrazine test
1–2 drops or 0.1g of O.S. + 2c.c. of reagent
Shake vigorously and heat if necessary– A yellow or orange ppt.
- Sodium bisulphite test
5-6 drops or 0.2 g of O.S. + 1 c.c. of saturated solution of sodium bisulphite-
Shake vigorously - A crystalline white ppt.
- Sodium Nitroprusside test
5-6 drops or 0.1 g of O.S. + 2 c.c. of 0.5 % aqs sodium nitroprusside solution + 2–3 drops of NaOH–A red or purple colour
Note - Benzophenone does not give this test. First two tests are also given by aldehydes
CARBOXYLIC GROUP (–COOH)
- Sodium bicarbonate test
Small amount of O.S. + 3 c.c. of saturated solution of sodium bicarbonate
Brisk effervescence
Brisk effervescence
- Litmus test
Place O.S. on moist blue litmus paper. If it changes to red the O.S. may be an acid
Note- Phenols also give this test
- Ester test
Small quantity of O.S. + 4-5 drops of drops of conc.  fruity smell
fruity smell
AMINO GROUP (–NH2, PRIMARY)
- Carbylamine test
2 drops of O.S. + 2 drops of of alcoholic caustic potash Intolerable offensive odour
Intolerable offensive odour
Aromatic or aliphatic amines give this test
- Nitrous acid test
0.2 g of O.S. + 10 c.c. of 
Add 10% aqs Brisk effervescence (aliphatic
Brisk effervescence (aliphatic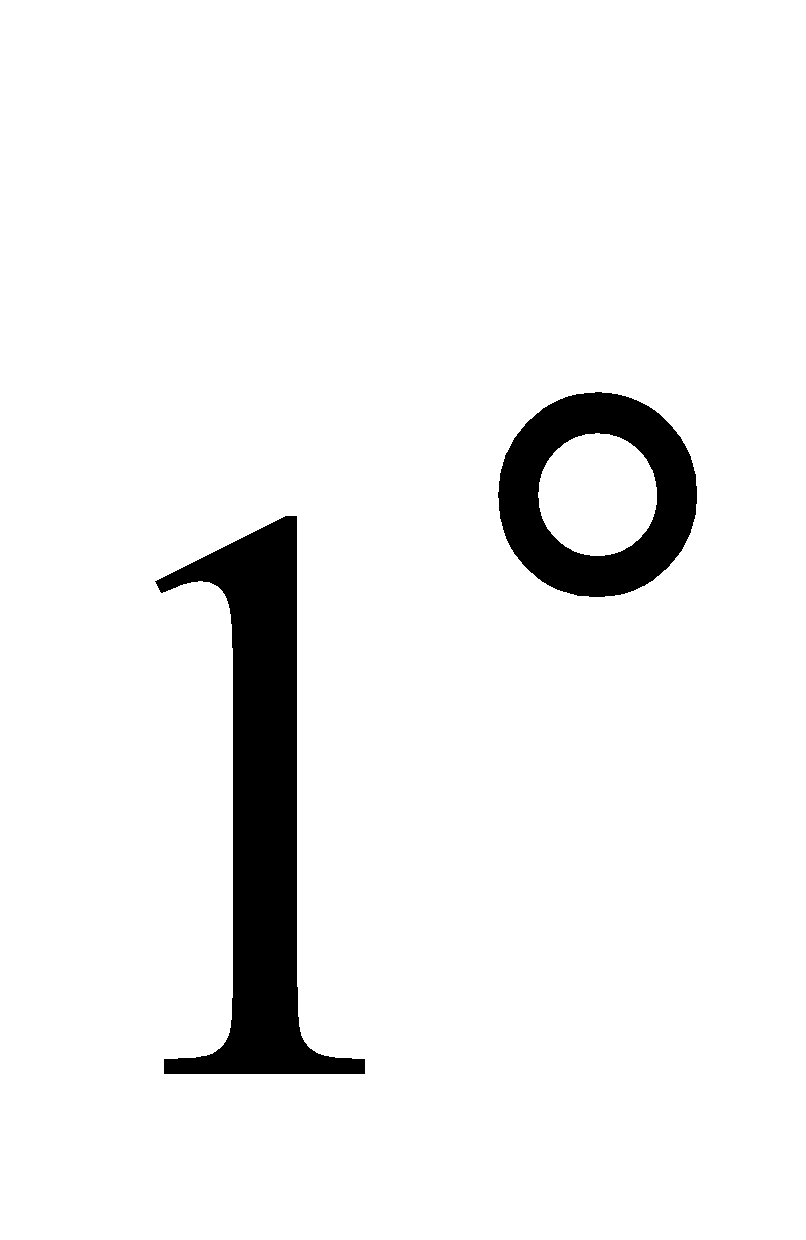 amine)
amine)
Add 10% aqs
- Azo-Dye test
This test is given by aromatic amines.
0.2 g or 0.2 ml of O.S. + 2 ml conc. HCl (cool) + 10% aqs NaNO2(cold) pour into a beaker containing 10% alkaline Bright Orange/red dye
- Distinguishing test for 1º, 2º and 3º amines (Hinsberg’s Test)
0.5 ml of O.S. + 2 ml of 25% NaOH + 1 ml of benzene sulphonyl chloride. Shake and Cool.
Precipitate dissolves in conc. HCl  3º amine
3º amine
Precipitate does not dissolve in conc. HCl  2º amine
2º amine
Insoluble mass is formed on addition of conc. HCl  1º amine
1º amine
- Solubility Test
Small amounts of O.S. + 2-3 ml dil HCl vigorously shake - If soluble, it may be an amine
RNH2 + HCl (R
(R H3) Cl–
H3) Cl–
Amines are basic in nature
- Litmus Test
Place O.S. on moist red litmus paper. If it is changes to blue the O.S. may be an amine.
CHEMISTRY INVOLVED IN PREPARATION OF INORGANIC COMPOUNDS
FERROUS AMMONIUM SULPHATE (MOHR’S SALT)
Reagents - Iron fillings, ammonium sulphate and conc. sulphuric acid.
Procedure - Take 60 c.c. of distilled water in a conical flask and add to 8 c.c. of conc. sulphuric acid. Gradually add 10 gm of iron filings and heat to boiling. Now add 20 gm of ammonium sulphate and evaporate to one third of its volume. Cool the flask slowly and set aside to crystallise.
Formula - 
POTASSIUM ALUMINIUM SULPHATE (POTASH ALUM)
Reagents - Potassium sulphate, Aluminium sulphate crystals
Procedure - 2.9 gm of potassium sulphate is dissolved in 30 c.c. of distilled water. 11.2 gm of aluminium sulphate is dissolved in 30 c.c. of distilled water. Mix the two solutions and leave overnight. Choose the well formed crystal and allow it to grow in the solution. Recrystallise by dissolving in minimum quantity of water.
Formula - 
CHEMISTRY INVOLVED IN PREPARATION OF ORGANIC COMPOUNDS
ACETANILIDE
Reagents : Aniline 10 ml
Acetic anhydride 10 ml
Acetic acid 10 ml
Zinc dust 0.05 gm
Procedure : Take 10 ml of aniline in 150 ml of conical flask and add a mixture of 10 ml of acetic anhydride and 10 ml of acetic acid. Add 0.05 gm of Zinc dust. Reflux using water condenser for 15 minutes. Pour the hot mixture in 200 ml of ice cold water with constant stirring. Filter the crystals of acetanilide formed and wash with cold water. Recrystallise from dil. acetic acid (20 ml glacial acetic acid + 40 ml water).
Formula : 
White shining flakes mpt 113ºC
IODOFORM
Reagents : Alcohol (5 gm); Sodium Carbonate (10 gm); Iodine (10 gm).
Procedure : 5 gm of ethyl alcohol and 10 gm of sodium carbonate in 50 ml of water are taken in round bottom flask. 10 gm of iodine is added in small fractions and the solution is stirred well and heated on water bath until the iodine has disappeared. Then cool, crystals of iodoform will separate. Wash them with distilled water
Formula : 
Bright yellow
ANILINE YELLOW
Reagents: Aniline 7ml
Conc. HCl 10ml
Sodium nitrite 2.5g
Sodium acetate 10.5g
Procedure: Take 7ml of aniline in 250ml of conical flask and add 10ml of Conc. HCl followed by 40ml of water and shake. Flask is placed on a water bath containing crushed ice. 2.5g of sodium nitrite dissolved in 10ml of water added gradually and stirred. When sodium nitrite solution has been completely added, keep the solution for 5 minutes in the water bath. Dissolve 10.5g of sodium acetate in 20ml of water and slowly added to the contents in the flask. Keep the flask in water bath for 20min and stirred. A yellow ppt. of aniline yellow is obtained. This yellow solid is washed with water and then dry between folds of filter paper. The crude dye is recrystallise busing light petroleum.
p-NITRO ACETANILIDE
Reagents : Acetanilide 5 g
Glacial acetic acid 5 ml
Fuming 2 ml
Conc. 10 ml
Methylated spirit 20 ml
Procedure : Take 5 g of powdered acetanilide in 100 ml conical flask. Add 5 ml of glacial acetic acid and shake. Take 2 ml of fuming nitric acid in a test tube and cool it in a freezing mixture. Add to it 2 ml of conc. sulphuric acid drop by drop with constant shaking and cooling. Add 8 ml of conc. drop by drop to the conical flask containing acetanilide and glacial acetic acid and place the flask in a freezing mixture. Add nitrating mixture to this flask drop by drop with constant stirring. Remove the conical flask from the freezing mixture and keep at room temperature for 15-20 minutes. Pour the contents of the flask on crushed ice in a beaker. Stir and filter the crude product. Crystallise the p-nitro acetanilide by dissolving it in methylated spirit and washing with cold water.
Formula : 
Colourless crystalline solid mpt
CHEMISTRY INVOLVED IN THE TITRIMETRIC EXERCISES
Volumetric or titrimetric analysis are quantitative analytical techniques which employ a titration in comparing an unknown with a standard. In a titration, a volume of a standardized solution containing a known concentration of reactant ‘A’ is added incrementally to a sample containing an unknown concentration of reactant ‘B’ till reactant ‘B’ is just consumed (stoichiometric completion). This is known as the equivalence point.
At this point we have N1V1 = N2V2.
Indicator : A compound added to the reacting solutions that undergo an abrupt change in a physical property usually a colour .
Types of indicators
- Internal - They are added to the reacting solutions
- External - Electrochemical devices such as pH meters
End point : The point at which a titration is stopped i.e. equivalence point.
Standard solution : The solution of known concentration of an acid, base or salt.
Examples of volumetric titrations
PREPARE A STANDARD SOLUTION M/100 OF OXALIC ACID. USING THIS SOLUTION DETERMINE THE MOLARITY OF THE GIVEN SOLUTION OF KMNO4
Theory - The reactions involved are
In this reaction oxalate ion is behaving as reducing agent and MnO4– ion as oxidising agent.
Oxidation half reaction
Reduction half reaction
MnO4– + 8H+ + 5e Mn++ + 4H2O
Mn++ + 4H2O
by using molarity equation we can calculate the molarity of solution.
n1 × M1 × V1 = n2 × M2 × V2
(KMnO4 solution) (oxalic acid)
where n1 = 5 and n2 = 2
Indicator - KMnO4 is self indicator
End Point - Appearance of light pink colour
Observation
Molarity of oxalic acid solution = M/100
Volume of oxalic acid solution = 25 ml
Calculation - n1 × M1 × V1 = n2 × M2 × V2
(KMnO) (oxalic acid)
Molarity of KMnO4 (M1) = 
PREPARE A STANDARD SOLUTION OF M/100 FERROUS AMMONIUM SULPHATE. USING THIS SOLUTION DETERMINE THE MOLARITY OF THE GIVEN SOLUTION OF KMNO4
Theory - The reactions involved are
The reaction shown above is a redox reaction and hence the titration between ferrous ammonium sulphate and KMnO4 is a redox titration.
In this reaction ferrous sulphate from Mohr’s salt is behaving as reducing agent and KMnO4 is acting as oxidising agent.
Oxidation half reaction -
Fe++ Fe+++ + e
Fe+++ + e
Reduction half reaction
MnO4– + 8H+ + 5e Mn++ + 4H2O
Mn++ + 4H2O
by using molarity equation we can calculate the molarity of solution.
n1 × M1 × V1 = n2 × M2 × V2
(KMnO4) (FeSO4)
Indicator - KMnO4 is self indicator
End Point - Appearance of light pink colour
Observation
Molarity of ferrous ammonium sulphate = M/100
Volume of ferrous ammonium sulphate = 25 ml
Calculation :
Molarity of KMnO4 (M1) =
CHEMICAL PRINCIPLES INVOLVED IN SOME EXPERIMENTS
TO DETERMINE THE HEAT OF NEUTRALISATION OF A STRONG ACID WITH A STRONG BASE
APPARATUS
Calorimeter (or a thermos flask)
Thermometer, Glass stirrer,
200 c.c. N NaOH
200 c.c. N HCl
Thermometer, Glass stirrer,
200 c.c. N NaOH
200 c.c. N HCl
THEORY
The neutralisation reaction of a strong acid with a strong base is essentially the combination of one equivalent of H+ ions with one equivalent of OH- ions
Equal volumes of the acid and alkali of the same strength at the same temperature are mixed in a calorimeter and the rise in temperature is noted. Water equivalent of calorimeter is determined. The heat of neutralisation is calculated.
PROCEDURE
- Determination of the water equivalent of calorimeter :
Take a definite weight of cold water in the calorimeter and note its temperature. Add to it a definite amount of hot water of known temperature and note the temperature of the mixture. The water equivalent of calorimeter (W) is determined by the following equation.
(Weight of cold water + water equivalent) (Final temperature of mixture - temperature of cold water)
= (Weight of hot water) (Temperature of hot water - temperature of the mixture)
- Take 100 c.c. of N NaOH in the calorimeter and add to it 100 c.c. of N HCl. Mix them well with the stirrer. Note the final temperature of the mixture.
OBSERVATIONS
- Water equivalent of the calorimeter
Weight of cold water = m1
Weight of hot water = m2
Temp. of cold water = t1° C
Temp. of hot water = t2° C
Temp. of mixture = t3° C
Water equivalent W is given by
(m1 + W)(t3 – t1) = m2 (t2 – t3 )
(m1 + W)(t3 – t1) = m2 (t2 – t3 )
- Heat of Neutralisation
Volume of acid = 100 c.c.
Volume of alkali = 100 c.c.
Initial temp. of acid = t° C
Initial temp. of alkali = t° C
Final temp. of the mixture = t1° C
Rise in temperature = (t1– t)ºC
The amount of heat produced on mixing acid and alkali
= (100 +100 + W) (t1 – t) = x calories
Here x is the heat of neutralisation
TO DETERMINE THE HEAT OF SOLUTION OF COPPER SULPHATE
THEORY
The amount of heat evolved or absorbed when one mole of substance (solid/liquid) is dissolved in large quantity of water such that further dilution does not make any heat. Change is known as heat of solution.
APPARATUS
Dewar’s vacuum flask, stirrer, thermometer physical balance, copper sulphate
PROCEDURE
- Determination of water equivalent of Dewar’s vacuum flask
Take a definite weight of cold water in a Dewar’s vacuum flask and note its temperature. Add to it a definite amount of hot water of known temperature and note the temperature of mixture. Determine the water equivalent by the following equation
(Weight of cold water+Water equivalent) (Final temperature of mixture - temperature of cold water)
= (Weight of hot water) (Temperature of hot water – temperature of mixture)
= (Weight of hot water) (Temperature of hot water – temperature of mixture)
- In second aperture again take a definite weight of cold water in Dewar’s vacuum flask and note its temperature. Add to it definite amount of copper sulphate and stir the solution till the entire copper sulphate dissolves.
Note down the final temperature of the solution
Initial temperature of water = T1º C
Initial weight of water = W1 g
Final temperature of copper sulphate solution = T2º C
Weight of copper sulphate = W2 g
Weight of copper sulphate = W2 g
Water equivalent of Dewar’s flask = W
CALCULATIONS
Total mass of solution = (W1 + W2)g
Rise in temperature = (T2 – T1)º C
Heat gained by flask = W(T2–T1) × 4.18 J
Specific heat of water = 4.18 J/gºC
Heat gained by solution = (W1 + W2)(T2–T1) × 4.18 J
Total heat change during dissolution of copper sulphate
(W2g) 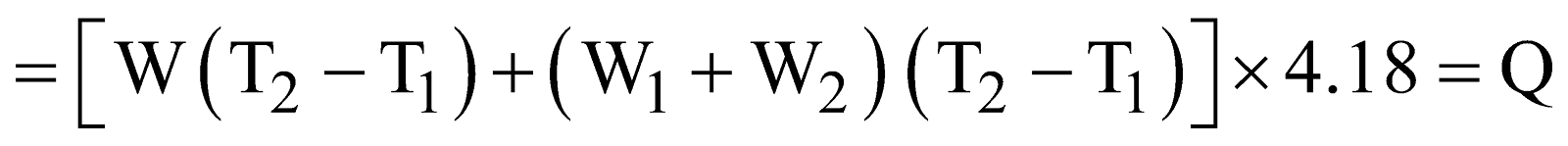
Total heat change during dissolution of 1 mole of copper sulphate will be
Where M is molecular weight of copper sulphate.
Hence heat of solution of copper sulphate
PREPARATION OF LYOPHOBIC AND LYOPHILIC SOLS
Preparation of arsenious sulphide solution
Boil about 1 - 2 g pure with about 500 ml of distilled water for about 10-12 minutes. Now cool the solution to room temperature and filter undissolved if any. Dilute this solution and pass into it gas (free from HCl by passing through a tube containing soda lime and then through 3-4 wash bottles containing distilled water) till it is saturated. Then pass a stream of pure hydrogen washed through 3-4 wash bottles containing distilled water to remove the excess of H2S. Filter the solution to remove the coarse precipitate of if necessary.
Preparation of Gelatin solution
Take about 400 ml of distilled water and heat it to 80-90℃. Add to it about 2g of powdered gelatin and dissolve it. Allow the solution to cool to room temperature. This gives a clear sol. of gelatin.
KINETIC STUDY OF IODIDE IONS WITH H2O2
Theory
In acidic medium, hydrogen peroxide (H2O2) oxidises iodide ions (I–) to iodine (I2) which is detected by starch solution.
H2O2+ 2I– + 2H+ 2H2O + I2
2H2O + I2
A known volume of sodium thiosulphate solution and starch solution is added to the reaction mixture for monitoring of reaction. I2 liberated react with sodium thiosulphate solution and reduced to iodide ions and when thiosulphate ions are totally consumed, the liberated iodine reacts with starch solution and gives blue colour, which detect the presence of iodine.
I2 + 2S2O23 –  S4O62– + 2I– Thiosulphate
S4O62– + 2I– Thiosulphate
I2+ starch solution  starch-iodine complex (Blue colour)
starch-iodine complex (Blue colour)
The time taken for the blue colour to first appear gives the idea about the rate of reaction.
Chemical required
0.1. M.KI soln., 2M HCl soln. Starch soln. 1M sodium thiosulphate sol. 4V H2O2 Soln.
Procedure
Take four different volume (apx. 25ml to 100ml ) of 0.1M KI soln. to four different flask and 50ml of 2M HCI is added to each flask. By dilution volume of soln. made upto 200ml and then 5ml starch soln., 2ml of 1M thiosulphate soln. and 25ml of H2O2 soln. is added to each flask.
Observation
Time required for the blue colour to first appear in :
Flask A — ................. seconds
Flask B — ................. seconds
Flask C — ................. seconds
Flask D — ................. seconds
Conclusion
It has been observed that time required for the blue colour to appear is decreases with increase in concentration of I– ions. Thus. “The rate of reaction increases with increase in concentration of iodide ions.”










