p-BLOCK ELEMENTS - CARBON FAMILY
GENERAL CHARACTERISTICS
ELECTRONIC CONFIGURATION
Element
|
Atomic No.
|
Electronic configuration
|
Valence shell configuration
|
Carbon
|
6
|
[He]
| |
Silicon
|
14
|
[Ne]
| |
Germanium
|
32
|
[Ar]
| |
Tin
|
50
|
[Kr]
| |
Lead
|
82
|
[Xe]
|
METALLIC CHARACTER
C and Si are non metals, Ge is a metalloid and Sn and Pb are metals.
APPEARANCE
C is black , Si is light-brown, Ge greyish white, Sn and Pb are silvery white.
DENSITY
Density increases with the increase in atomic number due to increase in mass per unit volume.
MELTING POINTS AND BOILING POINTS
The melting points and boiling points decrease from carbon to lead but carbon and silicon have very high melting and boiling points due to their giant structure.
OXIDATION STATES
C
|
Si
|
Ge
|
Sn
|
Pb
|
(+2) <+ 4
|
(+2 )<+ 4
|
+2 <+ 4
|
+2<+ 4
|
+2> + 4
|
- The compounds of Ge and Sn in +2 oxidation state are reducing in nature. Since their higher oxidation states +4 are more stable
- The compounds of Pb in +4 oxidation state are powerful oxidising in nature. Since +2 oxidation state of Pb is more stable
- The compounds in +2 oxidation state are ionic in nature and in +4 oxidation state are covalent in nature (Fajan’s rule)
NEGATIVE OXIDATION STATES
Carbon forms and in certain compounds e.g.
IONISATION ENERGY
It decreases from C to Sn . For Pb it is slightly higher than Sn.
ELECTRONEGATIVITY VALUES
The values decrease from C to Pb but not in a regular manner probably due to filling of d-orbitals in Ge and Sn and f-orbitals in Pb.
CATENATION
It is the tendency of an element to form long chains of identical atoms. The greater the strength of element-element bond, the greater is the strength of catenation.
Bond
|
C– C
|
Si–Si
|
Ge– Ge
|
Sn–Sn
|
Bond kJ/mole
|
353.3
|
225.7
|
167.2
|
155.0
|
ALLOTROPY
All the elements except Pb show allotropy.
Allotropic forms of carbon - Diamond, Graphite and Fullerene
Amorphous forms of carbon - coal, charcoal etc.
Silicon (Si) - crystalline and amorphous
Tin (Sn) - grey tin, white tin and rhombic tin
Germanium - two crystalline forms
VALENCY
All elements exhibit tetravalency. In case of Carbon 406 kJ/ mole of energy is required for promotion of 2s electron to 2p. Formation of two extra bonds provide this energy .
INERT- PAIR EFFECT
On descending the group, the stability of +4 oxidation state decreases and that of +2 oxidation state increases.
ATOMIC AND IONIC RADII
Both increase from C to Pb
ATOMIC VOLUME
Atomic volume shows a regular increase from C to Pb.
FORMATION OF COMPLEXES
C does not give any complex due to non availability of empty d orbitals in valence shell.
The valence shell of Si and other elements contain d-orbitals and can accomodate more than 8 and can therefore form complexes. e.g.,
The hybridisation in these complexes is which is octahedral.
REACTIVITY
Increases from C to Pb.
MULTIPLE BONDING
Carbon forms p - p
- p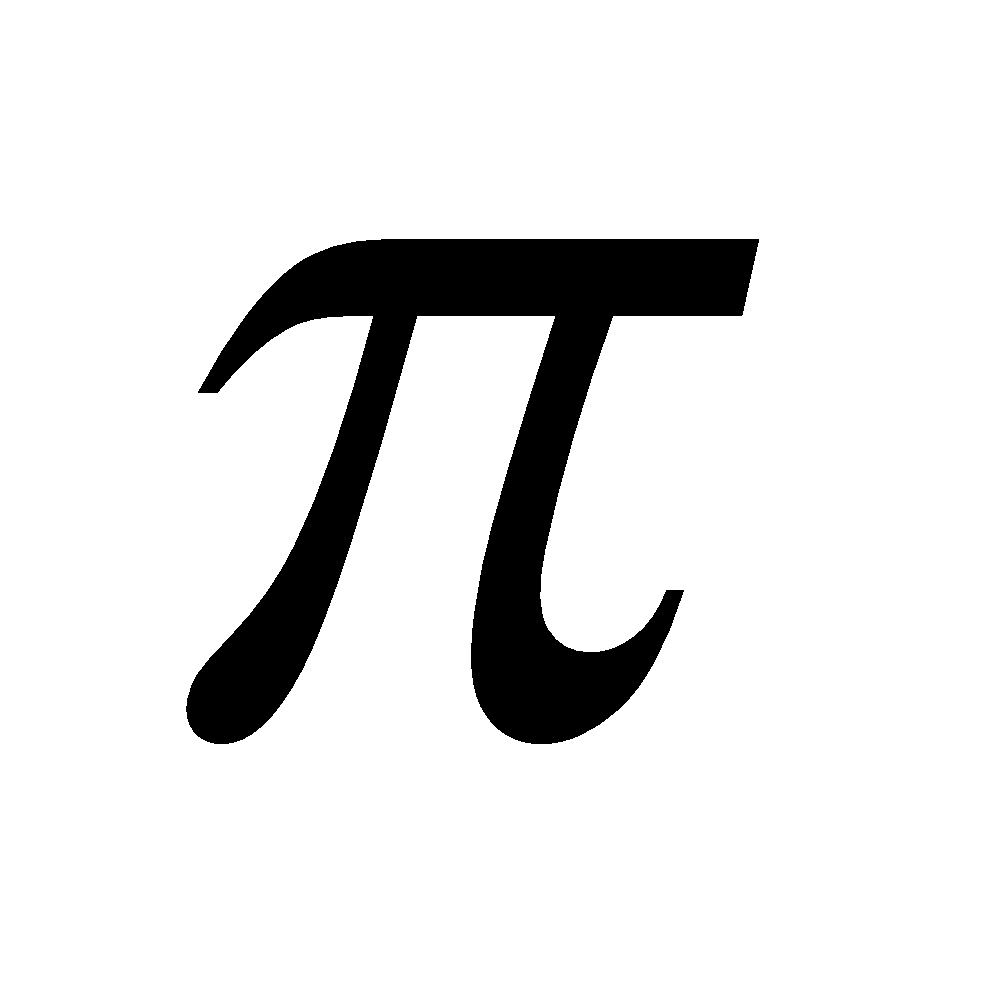 multiple bonds with itself and with S, N and O. Other elements show negligible tendency of this type due to their large size. Others form d
multiple bonds with itself and with S, N and O. Other elements show negligible tendency of this type due to their large size. Others form d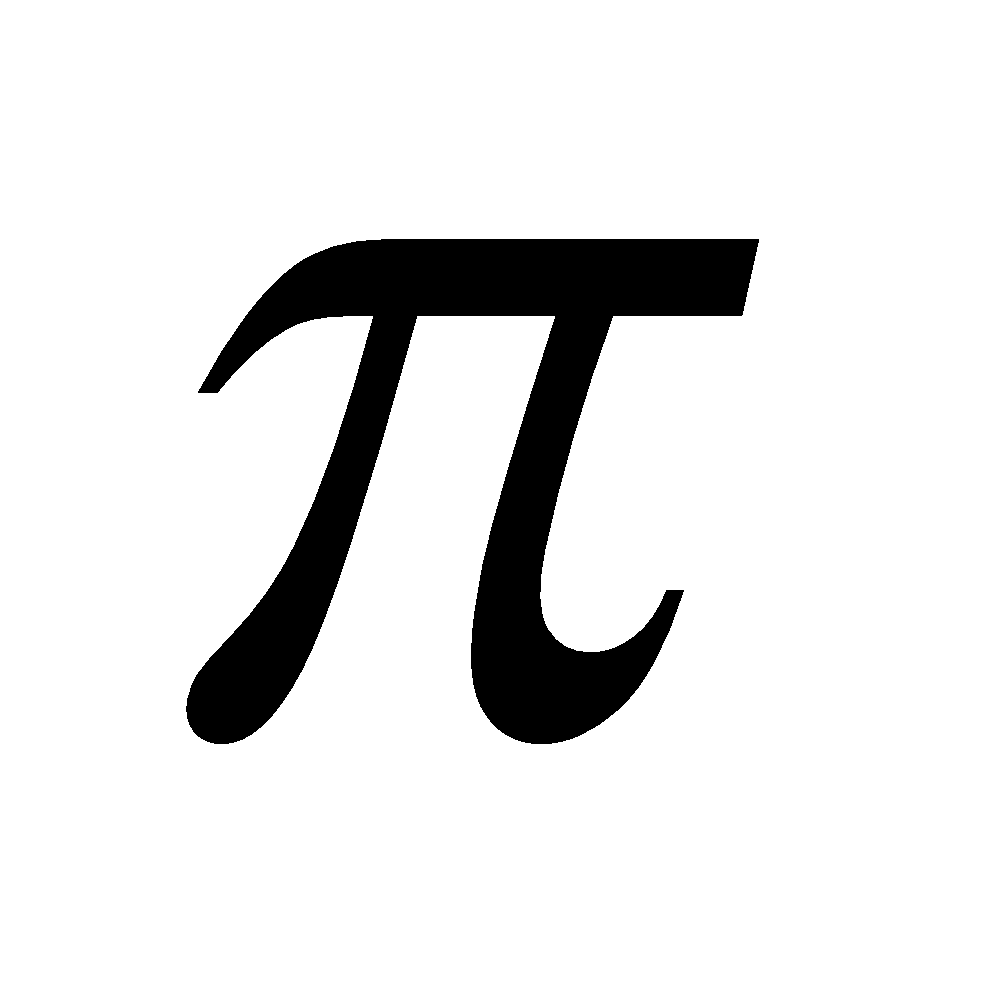 - p
- p multiple bonds.
multiple bonds.
FORMATION OF COMPOUNDS
HYDRIDES
All form covalent hydrides .Their number and ease of formation decreases down the group.
- Hydrides of carbon are known as Alkanes, Alkene or Alkynes.
- Hydrides of Si and Ge are known Silanes and Germanes but their number is limited.
- The only hydrides of Sn and Pb are SnH4 (Stannane) and PbH4 (Plumbane) .
- Their thermal stability decreases down the group.
- Their reducing character increases down the group.
HALIDES
All the elements give tetrahedral and covalent halides of the type except
except  and
and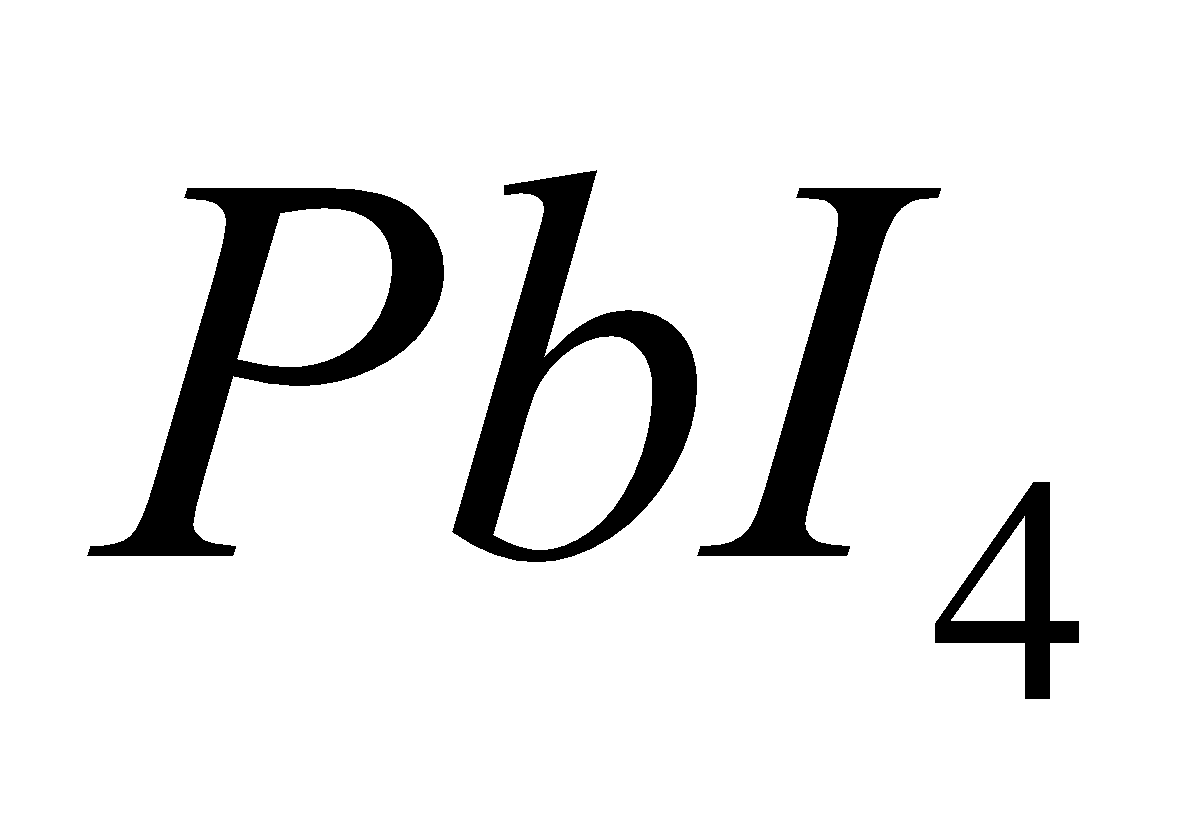 , since
, since  is strong oxidising and
is strong oxidising and 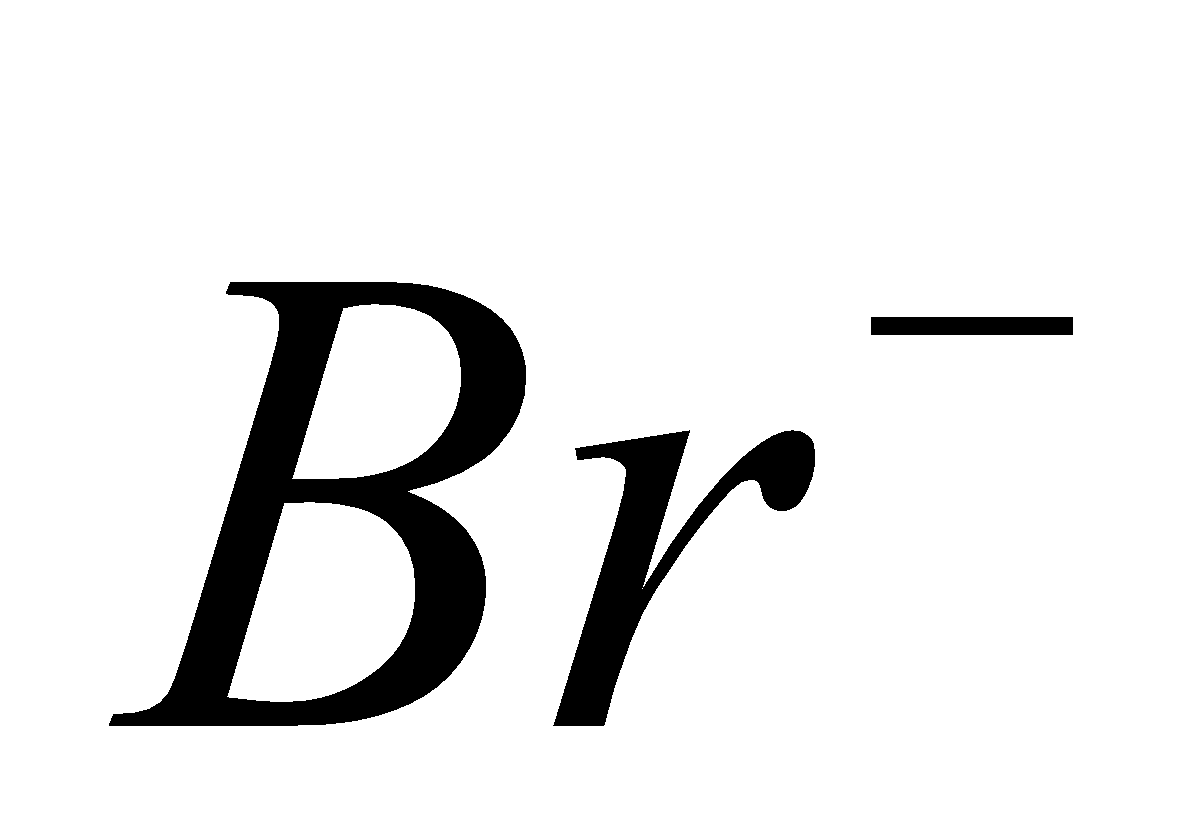 and
and 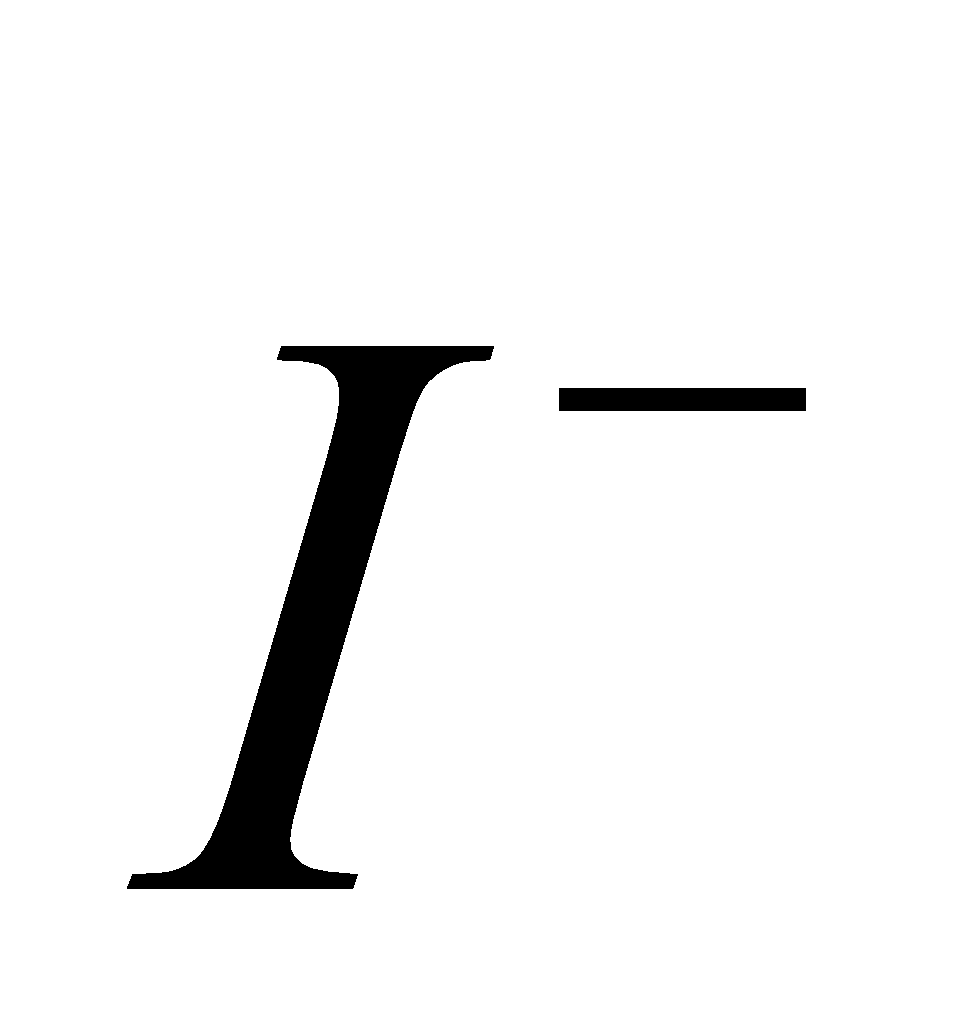 are strong reducing agent.SnF4 is ionic.
are strong reducing agent.SnF4 is ionic.
- Stability - Order of thermal stability with common halogen
Order of thermal stability with common metals
- Hydrolysis - Except other tetrahalides are hydrolysed
Ease of hydrolysis 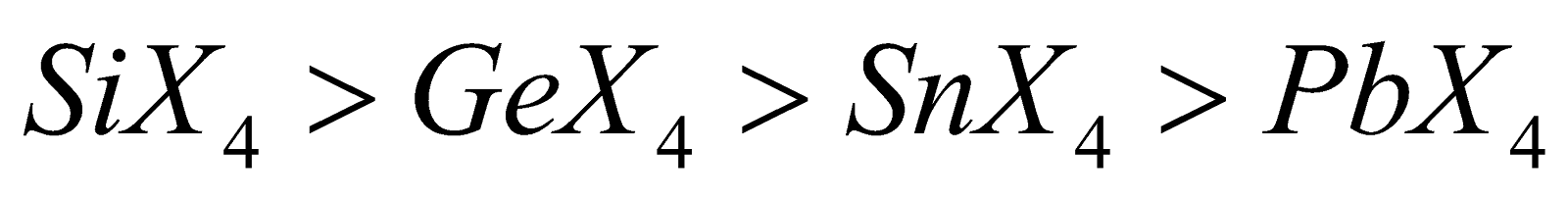
DIHALIDES
Except carbon other elements form dihalides of the type 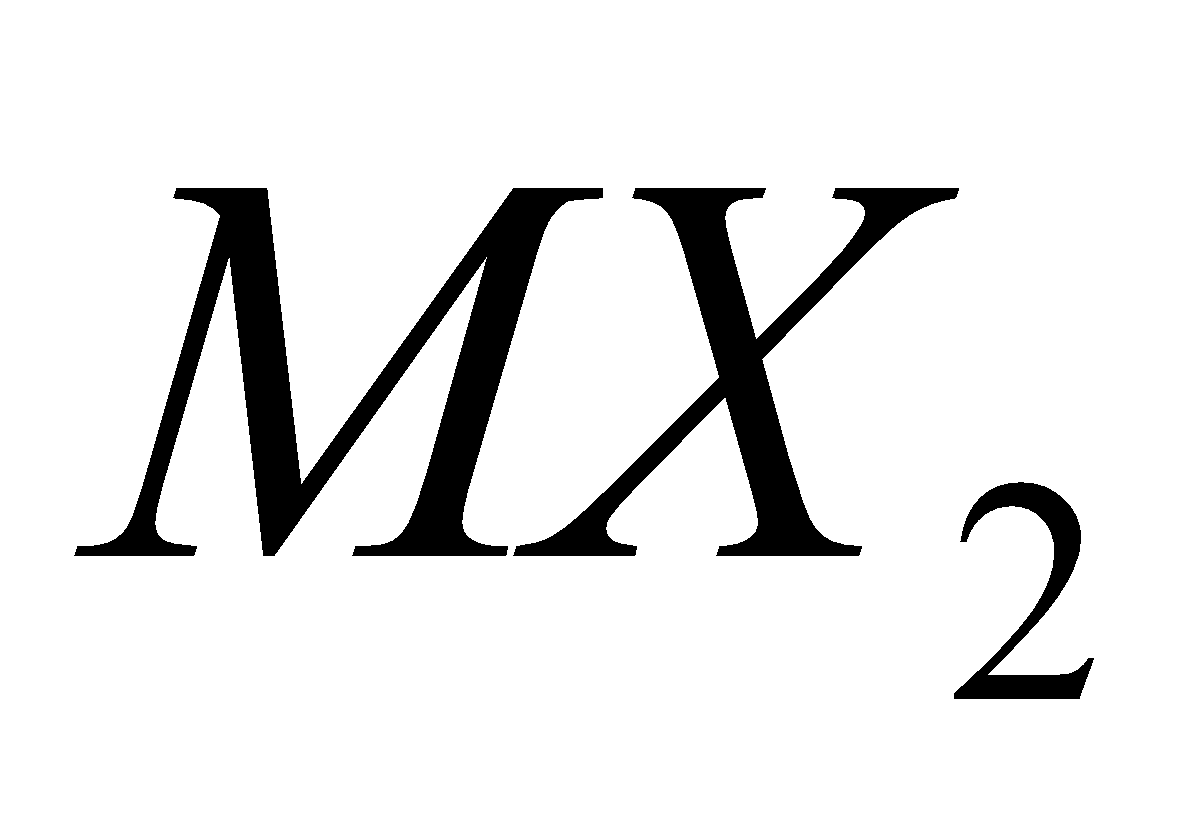 which are more ionic and have higher melting points and boiling points e.g
which are more ionic and have higher melting points and boiling points e.g 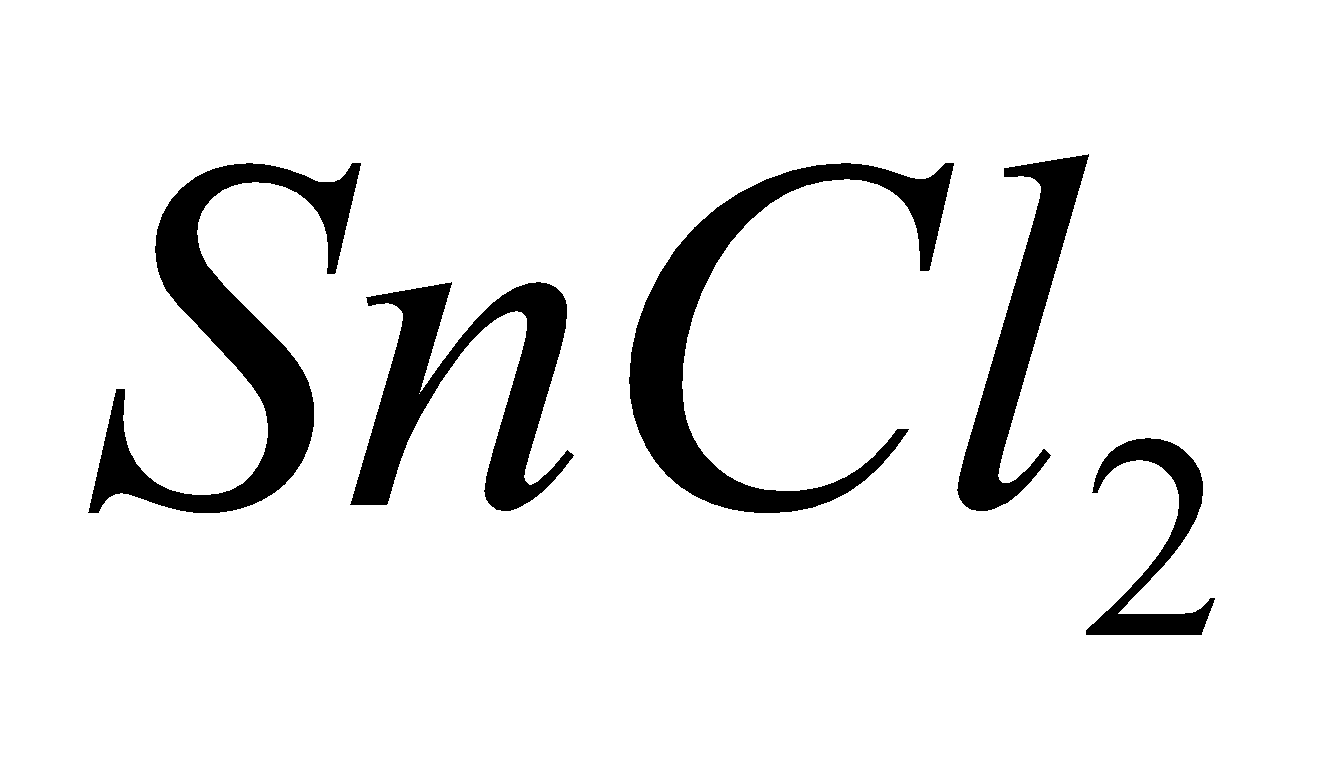 is a solid whereas
is a solid whereas  is a liquid at room temperature.
is a liquid at room temperature.
C, Si and Ge form trihalides of the type  . Pb and Sn do not form trihalides of the type
. Pb and Sn do not form trihalides of the type 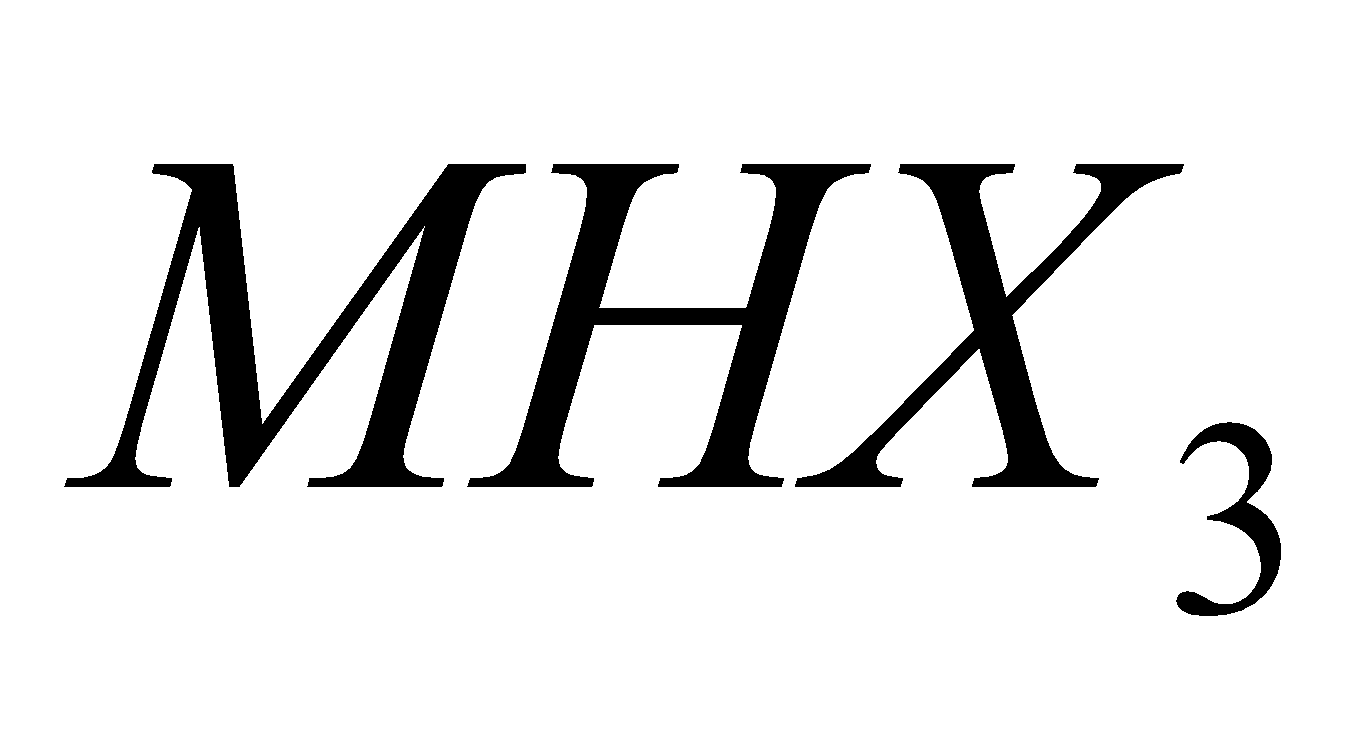
OXIDES
They form two types of oxides
Mono-oxides of the type MO
CO forms a number of coordination compounds with transition metals e.g. Ni(CO)4, Fe(CO)5 and Cr(CO)6
Dioxides of the type MO2
Acidic - CO2, SiO2
Amphoteric - GeO2, SnO2 and PbO2
The bond energy of 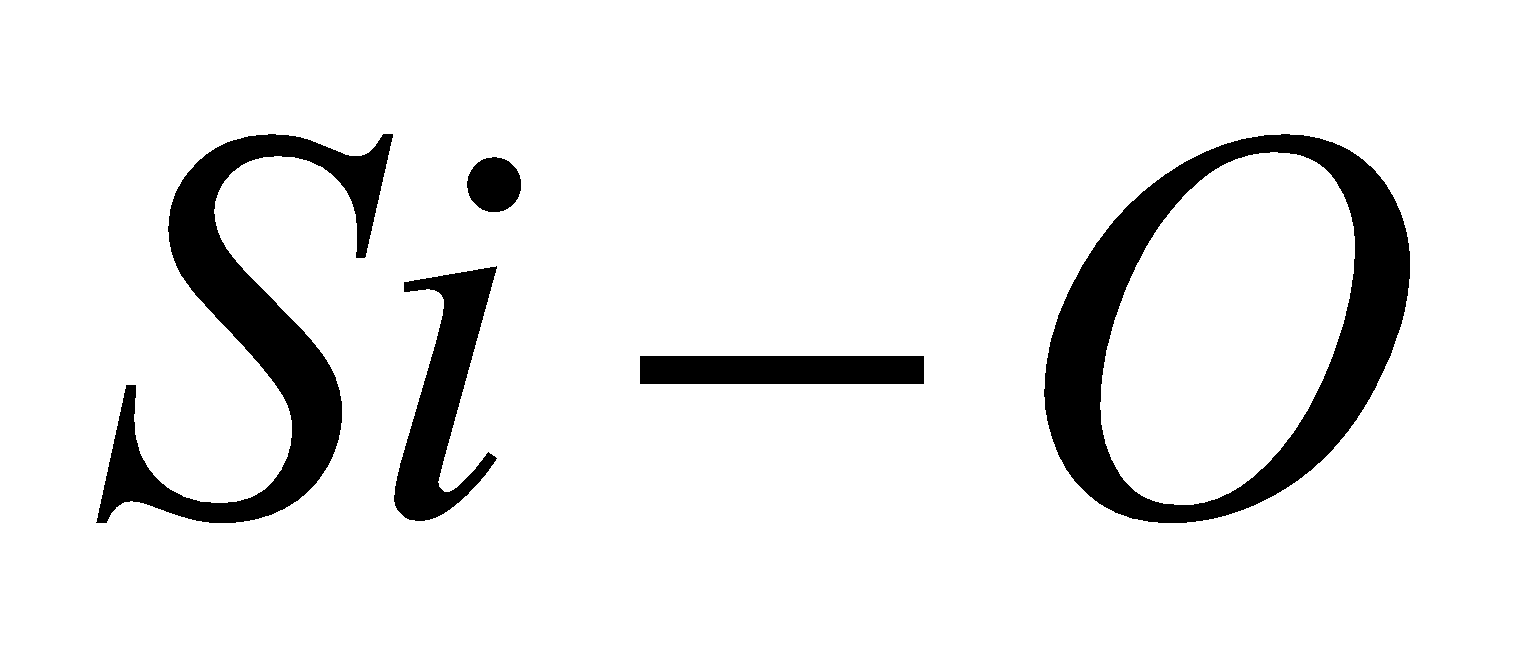 bond is 368 kJ/mol, therefore
bond is 368 kJ/mol, therefore  is chemically inert and has high melting point.
is chemically inert and has high melting point. 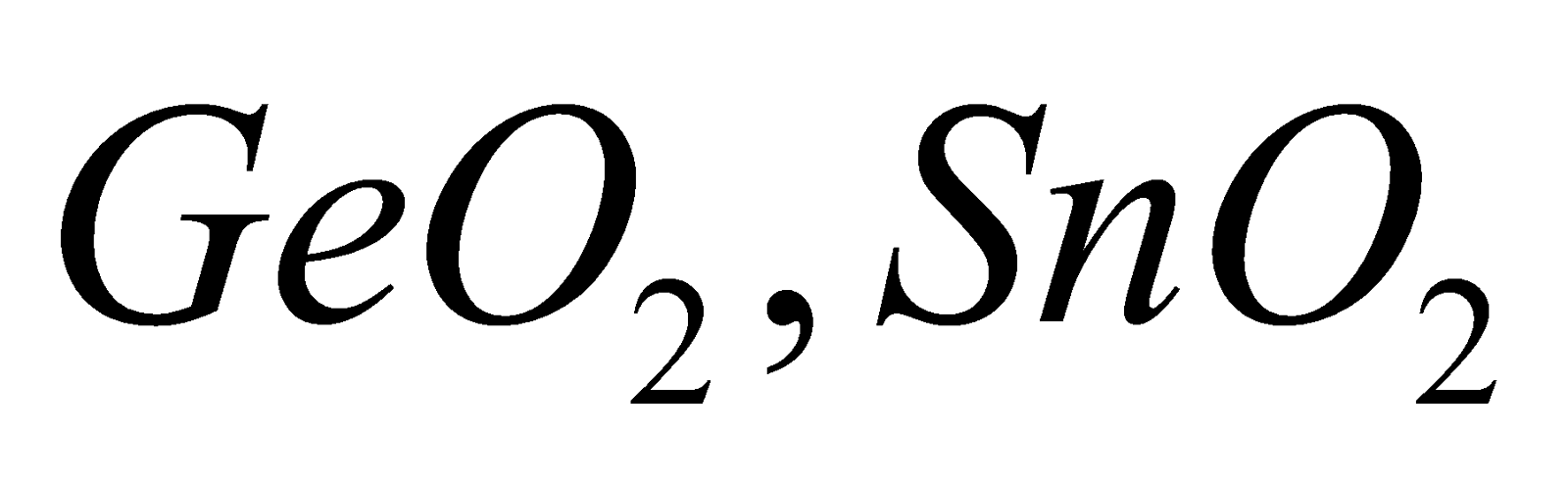 and
and 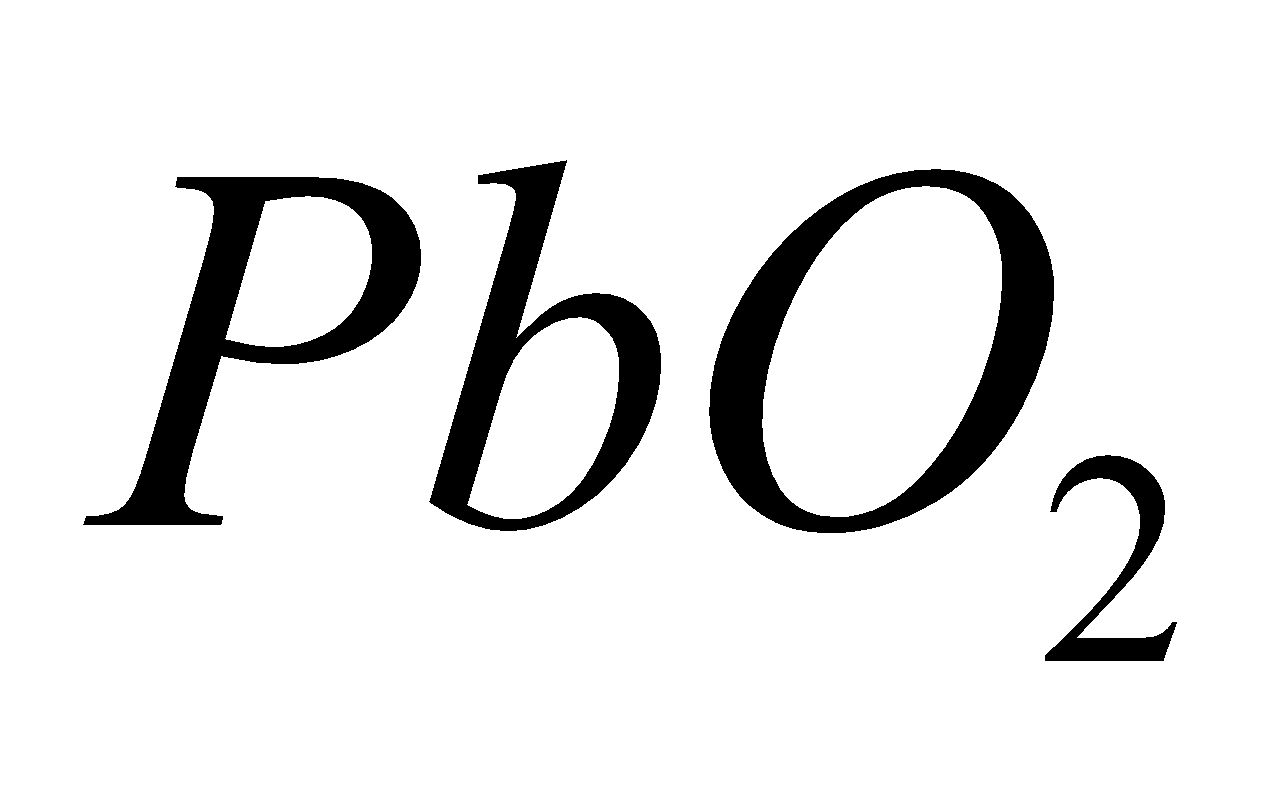 all are network solids.
all are network solids. 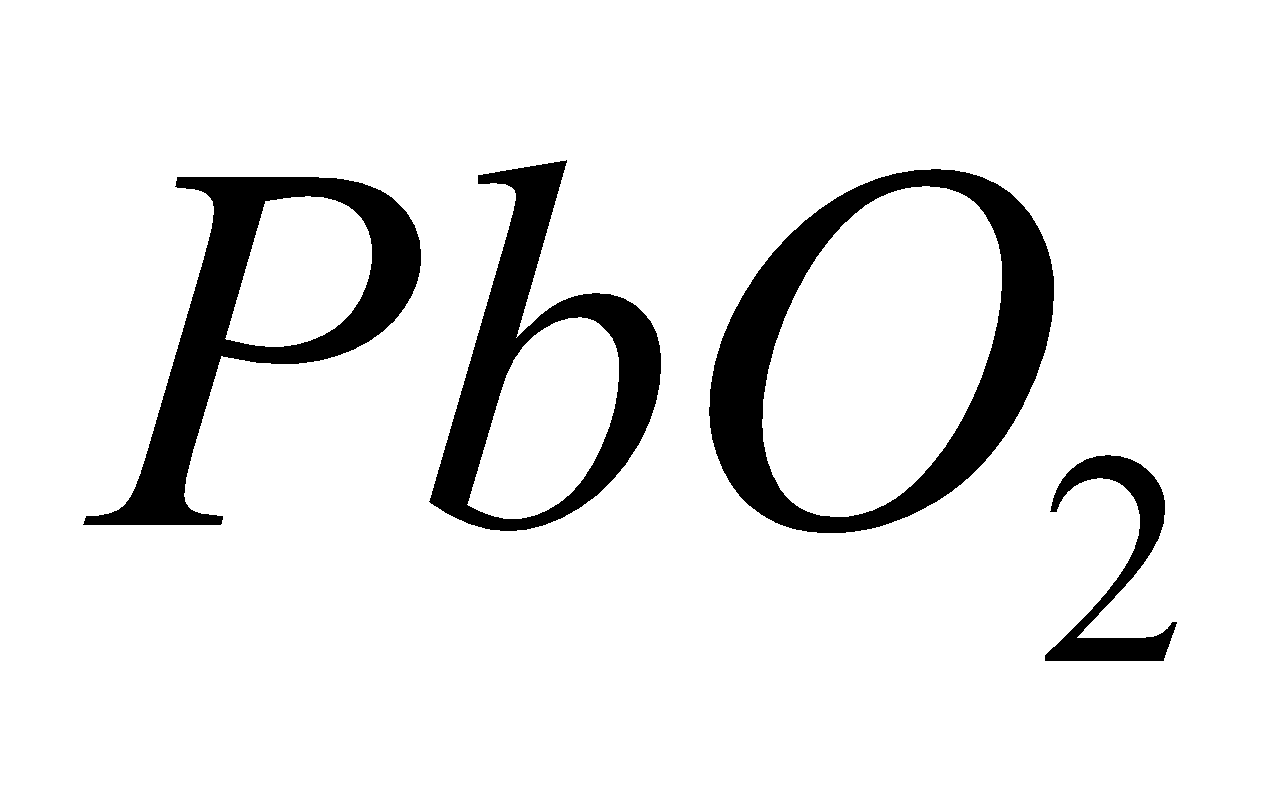 is a powerful oxidising agent
is a powerful oxidising agent
Carbon also gives suboxide
Lead also gives mixed oxide
ACIDS
All elements give acids of the type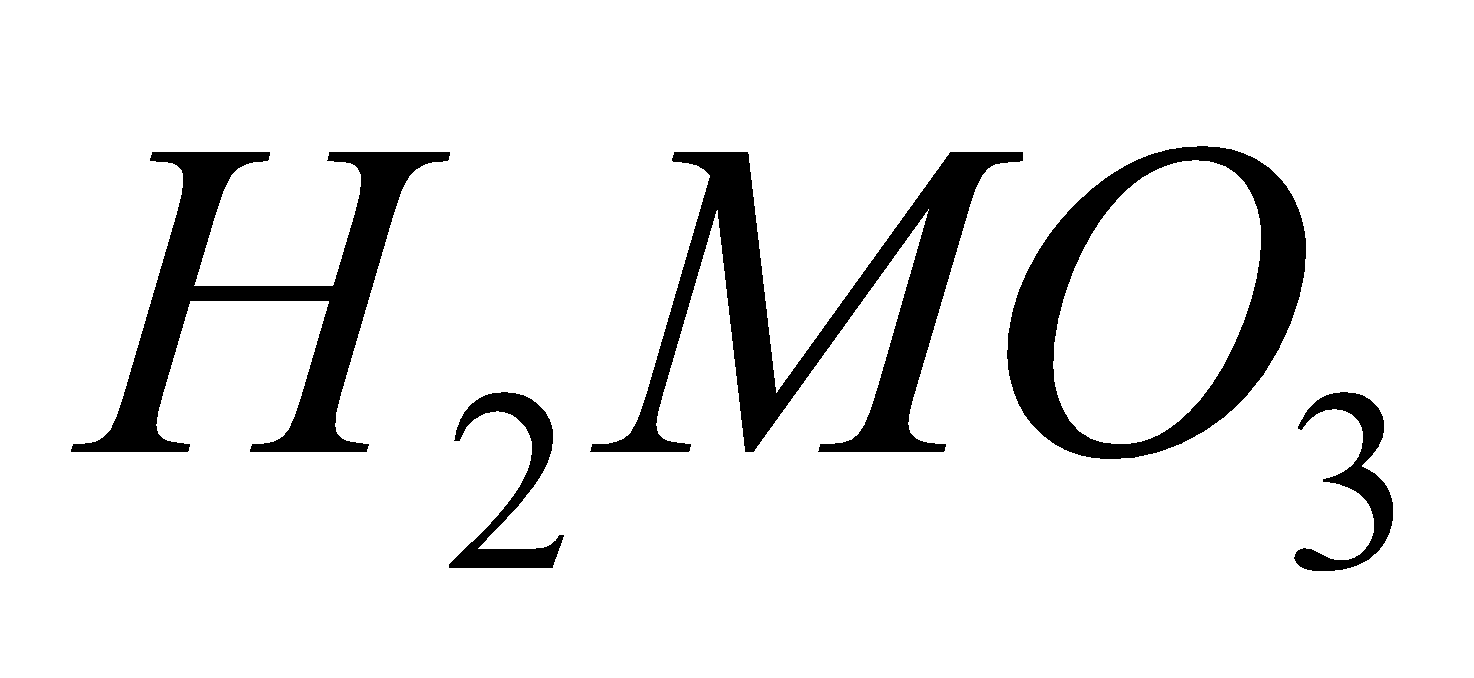 e.g.
e.g.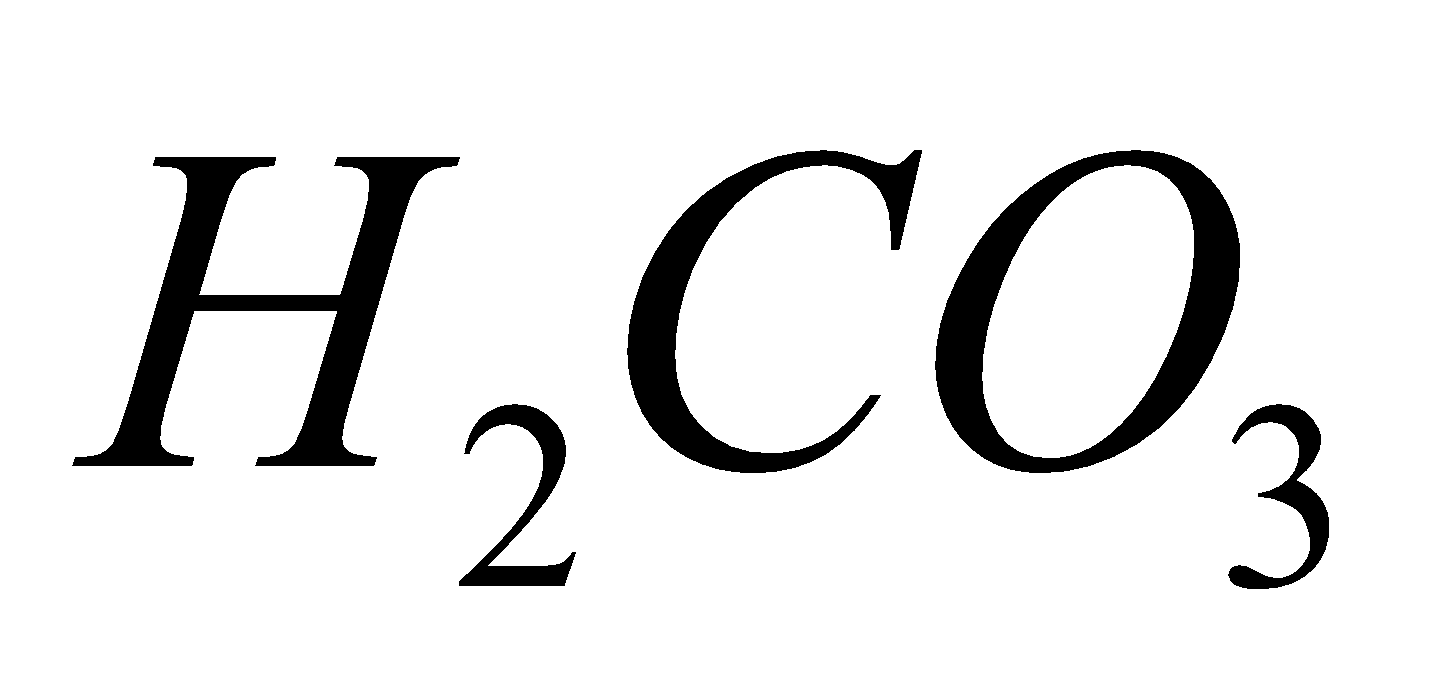 (carbonic acid),
(carbonic acid), (silicic acid),
(silicic acid), 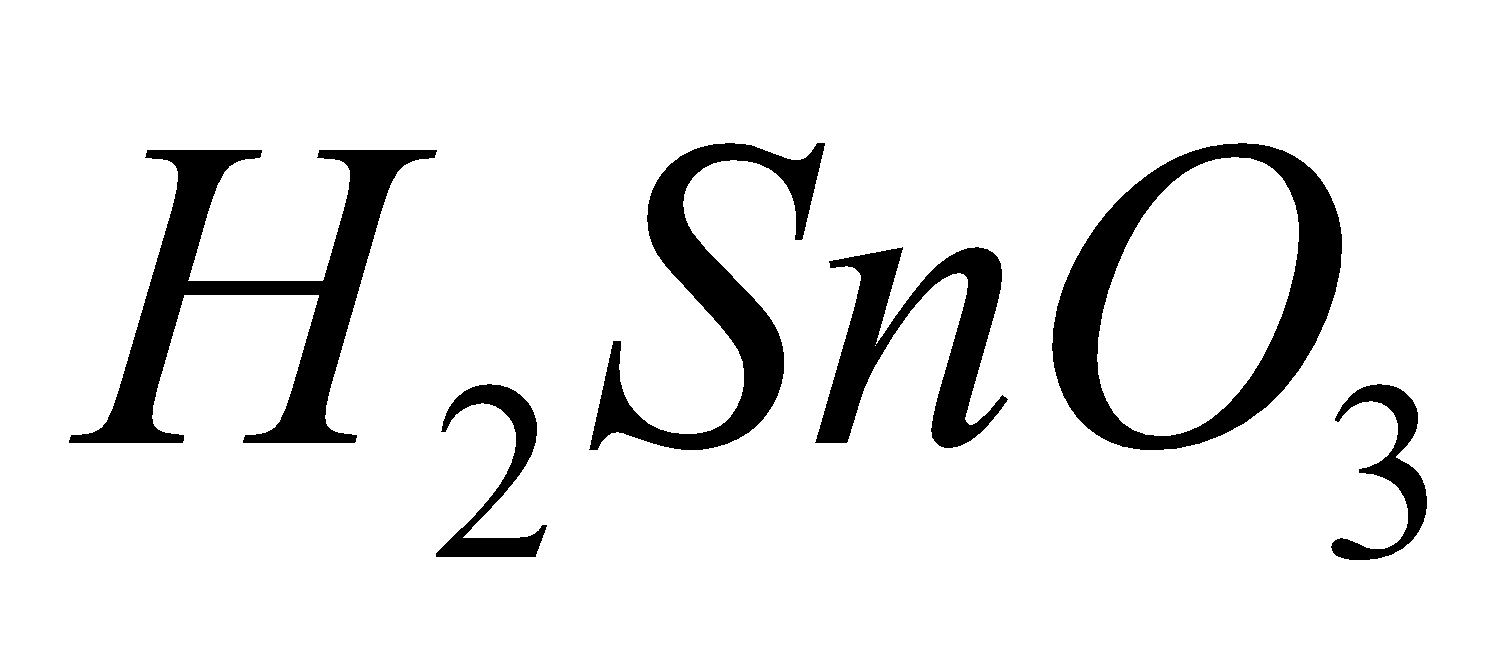 (metastannic acid),
(metastannic acid),  (meta plumbic acid). Carbonic acid forms two series of salts, bicarbonates
(meta plumbic acid). Carbonic acid forms two series of salts, bicarbonates 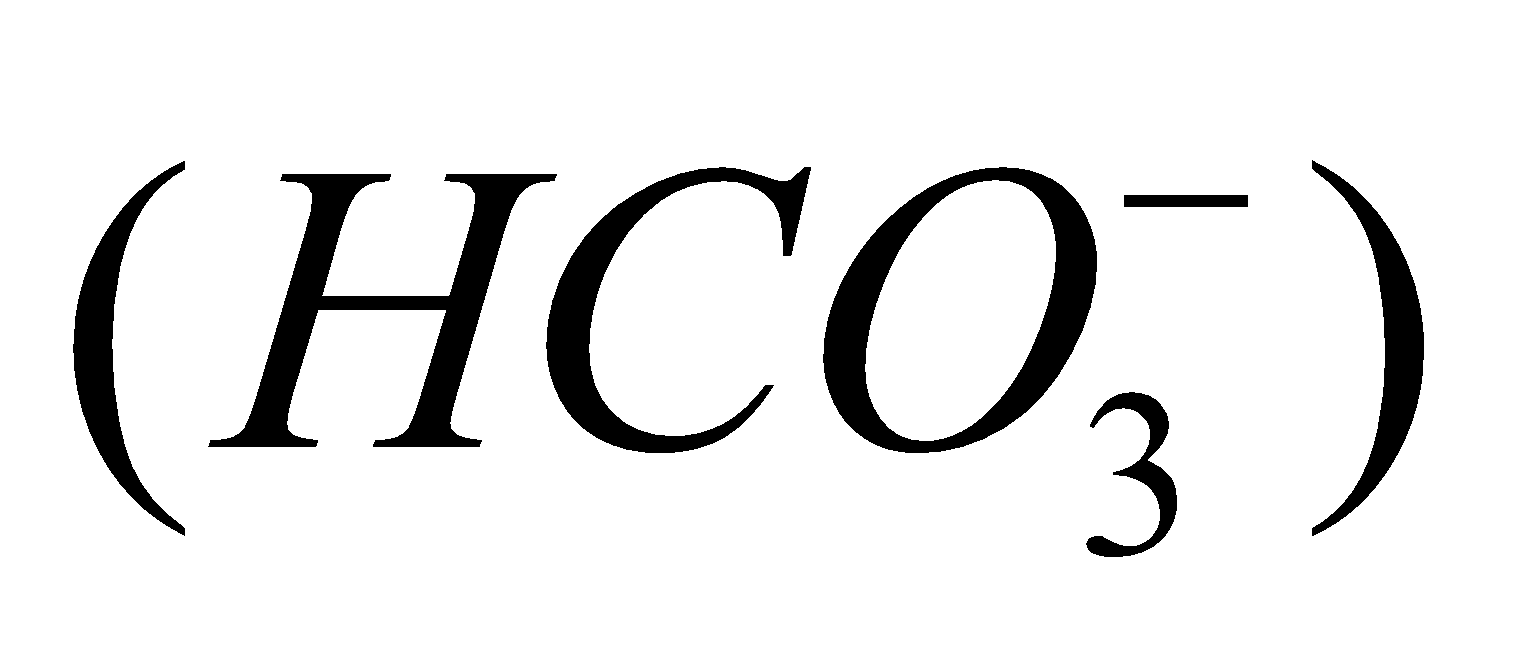 and carbonates
and carbonates .
.
SILICATES
Silicates are metal derivatives of silicic acid 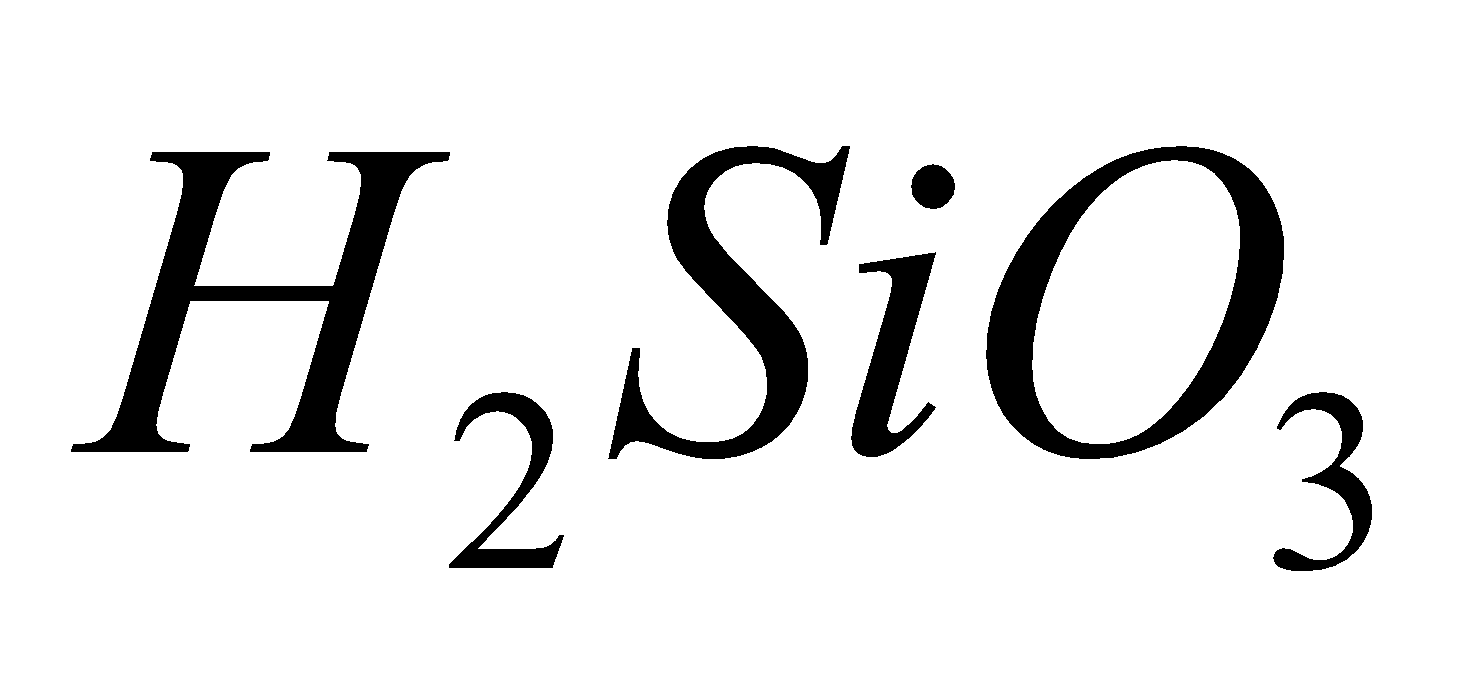 and can be obtained by fusing metal oxides or metal carbonates with sand e.g.
and can be obtained by fusing metal oxides or metal carbonates with sand e.g.
TYPE OF SILICATES
Silicates contain tetrahedral formed by  hybridisation, depending upon the number of O-atoms shared between tetrahedra and fashion, Silicates have been classified into following groups
hybridisation, depending upon the number of O-atoms shared between tetrahedra and fashion, Silicates have been classified into following groups
- Orthosilicates - They contain discrete
tetrahedra, Examples are phenacite
, willimite
forsterite
.
- Pyrosilicates - Here two tetrahedra units are
joined by one oxygen atom forming a large discrete. Examples are thortveitite
, hemimorphite
- Chain silicates - Here two oxygen atoms per SiO4 tetrahedra are shared giving polymeric anion chains. Discrete unit is
. Examples: synthetic sodium silicate,
lithium silicate
, natural spodumene
, jadeite
, enstatite
and diopside
.
- Double chains - Here two simple chains are held together by shared oxygen atoms. The discrete unit is
. Example mineral tremolde
Double chains silicates is also called amphibole
- Cyclic silicates - Here two oxygen atoms per
tetrahedra are shared giving discrete unit
and
. Example Beryl
- Sheet-silicates - Here three oxygen atoms per tetrahedra are shared giving two dimensional sheet having discrete unit
. Example
Talc  Kaolin
Kaolin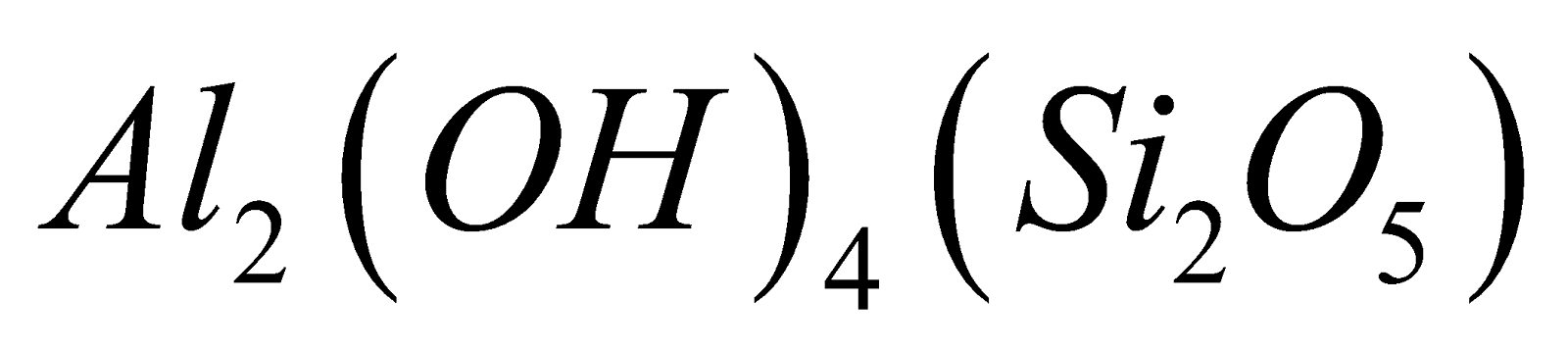 .
.
- Framework silicates - Here all four oxygen atoms of each tetrahedra are shared. Example are quartz, zeolites, tridymite and cristobalite.
SILICONES
The polymeric compounds containing units, linear cyclic or cross linked are known as silicones. They are manufactured from alkyl substituted chlorosilanes
Silicone
Silicones are chemically inert, water repellent, heat resistant, good electrical insulators. These are used as lubricants, insulators etc.
CARBIDES
Compounds of carbon with less electronegative elements e.g. Be, B, Si etc are called carbides. These are of three types.
IONIC OR SALT LIKE
The carbides of elements of group 1, 2, 13, coinage metals, Zn,Cd. Some lanthanides give ionic or salt like carbides. Prepared by heating oxide with carbon or hydrocarbon at high temperature (2350K)
They are further classified as
- Acetylides contain
. These liberate acetylene on hydrolysis
They have NaCl type crystal lattice.
- Methanides
- These react with water to give methane.
- Allylides - These react with water to give allylene.
INTERSTITIAL CARBIDES
These carbides are formed by transition elements especially Cr, Mn and Fe group metals. These are very hard.
COVALENT CARBIDES
Carbides of B and Si, B4C and SiC are covalent .SiC is known as CARBORUNDUM, used as abrasive and refractory material. B4C harder than SiC and used as an abrasive.
CARBON
It is widely distributed in the free state (diamond, graphite, coal etc.) and in the combined state (oxides, carbonates hydrocarbons etc.)
ALLOTROPIC FORMS OF CARBON
The crystalline forms include
- Diamond
It is beautiful crystalline form, hardest, and has three dimensional polymeric structure, hybridisation of C is  It is covalent solid, melting point 3650°C, density and bad conductor of heat and electricity.
It is covalent solid, melting point 3650°C, density and bad conductor of heat and electricity.
When heated at 1800°C - 2000°C, it is converted to graphite.
- Graphite
It is dark grey, having hexagonal plates, hybridisation of C is , good conductor of heat and electricity due to free movement of electrons. It was also known as black lead or plumbago. It is very good lubricant.
Aqua dag - suspensions of graphite in water
Oil dag - suspension of graphite in oil lubricants
- Fullerene
Fullerenes are large cage like spheroidal molecules with general formula C2n
(where n≥ 30). Two important member are C60 and C70. C60 fullerene looks like a soccer ball (so called bucky ball).
(where n≥ 30). Two important member are C60 and C70. C60 fullerene looks like a soccer ball (so called bucky ball).
AMORPHOUS FORMS OF CARBON
- Coal
- Coke
- Charcoal or wood charcoal
- Bone-black or animal charcoal
- Lamp-black
- Carbon-black
- Gas carbon
- Petroleum coke
Varieties of coal -
- Peat 60% carbon
- Lignite 70%C
- Bituminous 78%C
- Semi bituminous 83%C
- Anthracite 90%C
Anthracite is purest - amorphous form, burns without smokey flame.
Coke - Coal  Coke.
Coke.
It contains C = 80-90%
Uses - Reducing agent in Iron and steel industry for making water gas and graphite.
Wood charcoal - It is obtained by strongly heating wood without access to air. When heated with steam it becomes more activated.
Uses -To remove colouring matters and odoriferous gases.
Bone-black or animal charcoal - It is obtained by destructive distillation of bones in iron retort. By products are bone oil or pyridine.
Uses - As adsorbent. On burning it gives bone ash which is calcium phosphate and used in the manufacture of phosphorus and phosphonic acid.
Lamp black - It is obtained by burning vegetable oils in limited supply of air.
Uses - In the manufacture of printing ink, black paint, varnish and carbon paper.
Carbon black - It is obtained by burning natural gas in limited supply of air.
Uses - Added to rubber mixture for making automobile tyres.
Gas carbon and petroleum coke - When coal is subjected to destructive distillation carbon deposited on walls is scraped and called gas carbon. Similarly petroleum coke is deposited while distilling crude petroleum.
Uses - Both are good conductors of electricity when pressed into sticks they make good electrodes, known as gas electrodes.
Sugar charcoal - It is obtained by heating sugar in absence of air. It is purest form of carbon.
CARBON DIOXIDE CO2
PREPARATION
Lab method
Manufacture
- Fermentation
- From fuel gases
PHYSICAL PROPERTIES
Colourless, 1.5 times heavier than air, can be poured downwards like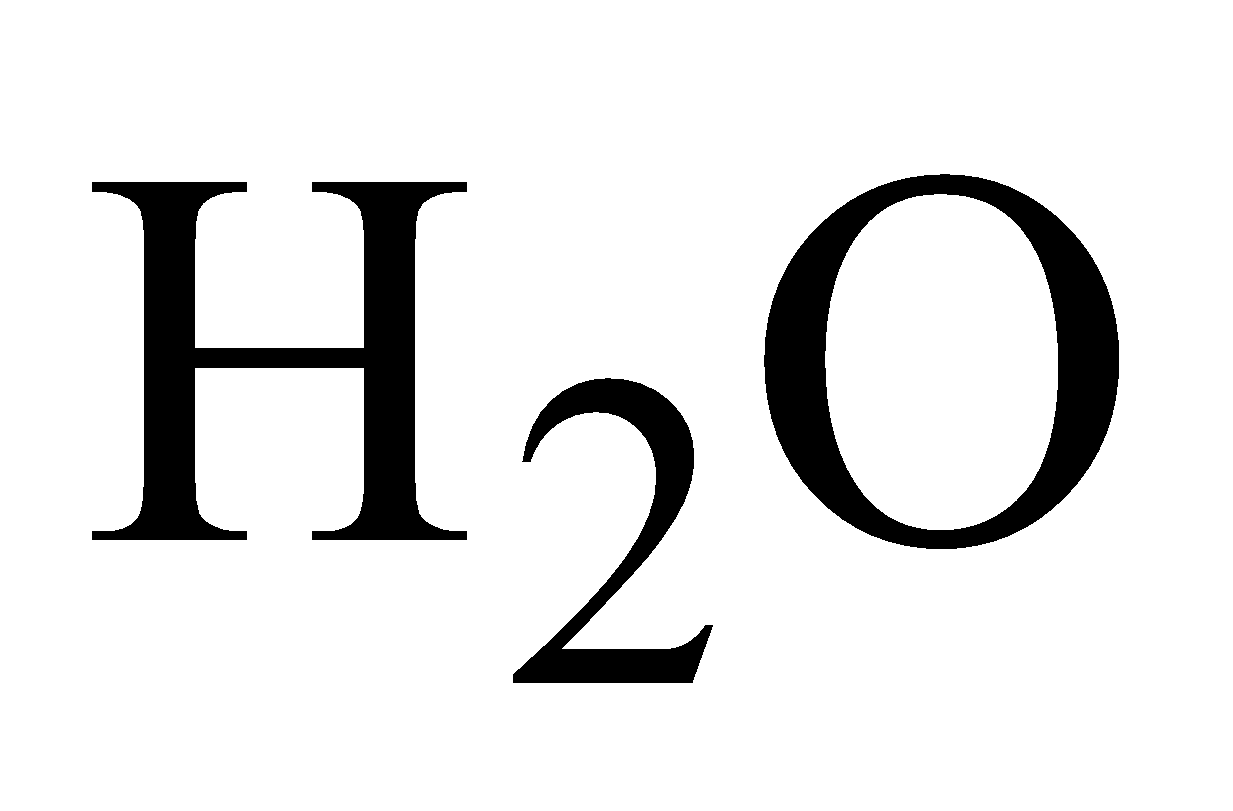 .Animals die in its presence due to lack of
.Animals die in its presence due to lack of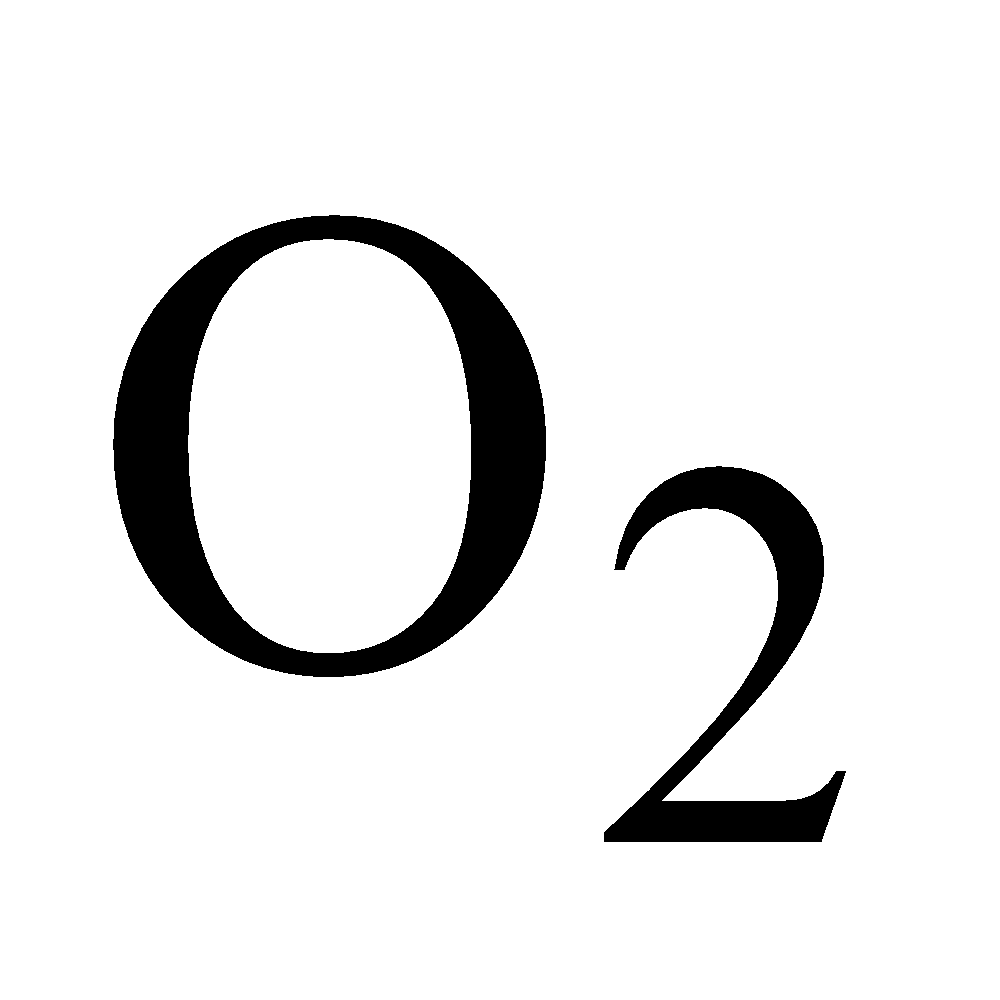 , it is also known as black damp.
, it is also known as black damp.
CHEMICAL PROPERTIES
- Stability - Fairly stable, decomposed at 1775K.
- Incombustible and non supporter of combustion but active metals e.g. Mg, Na, K continue burning in a jar of the gas.
- Acidic nature
- Lime water
- Reduction
- Photosynthesis
USES
In household as fire extinguisher. Dry powder fire extinguisher contains 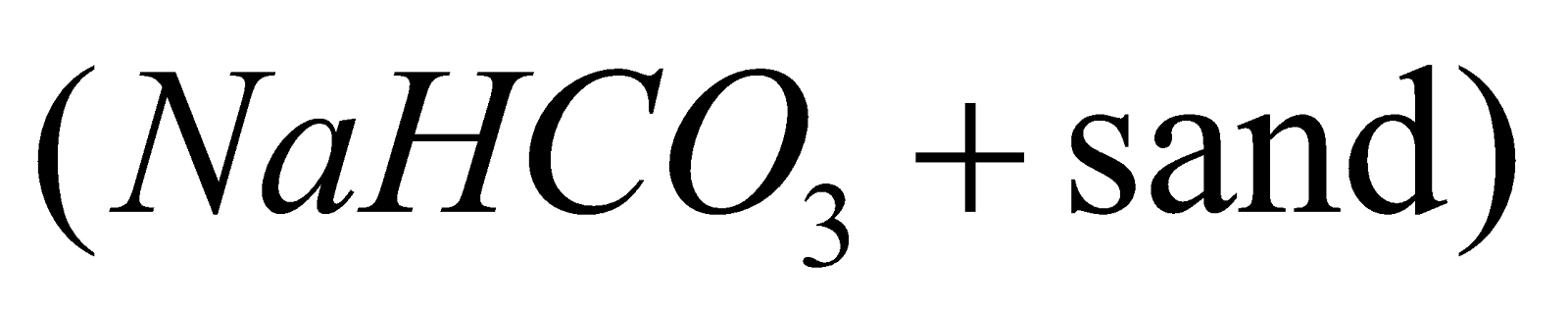 which is decomposed by heat.
which is decomposed by heat.
Foamite extinguisher contains baking soda and aluminium sulphate and is used for oil fires.
Structure .  Linear, dipole moment is zero.
Linear, dipole moment is zero.
CARBON MONOXIDE CO
PREPARATION
Lab method
Manufacture
(a) Air 
(b) Steam 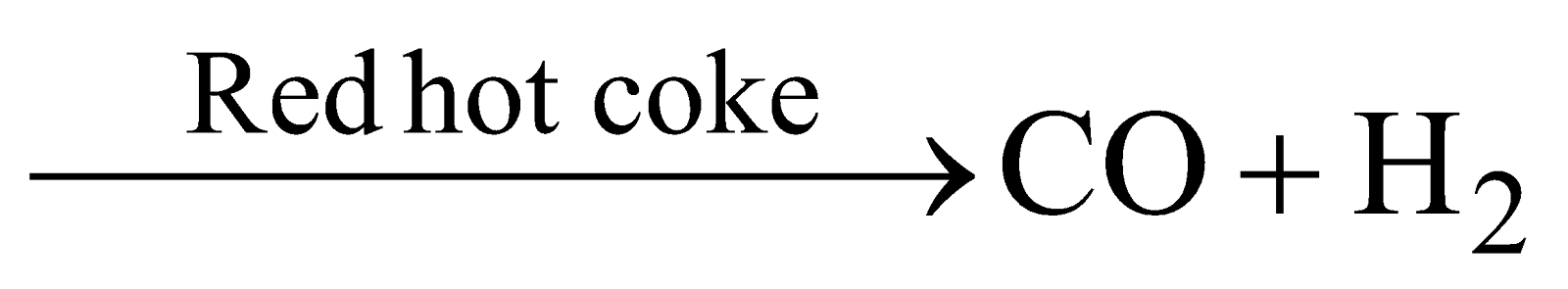 (water gas synthesis gas or blue gas )
(water gas synthesis gas or blue gas )
Other methods
Heating 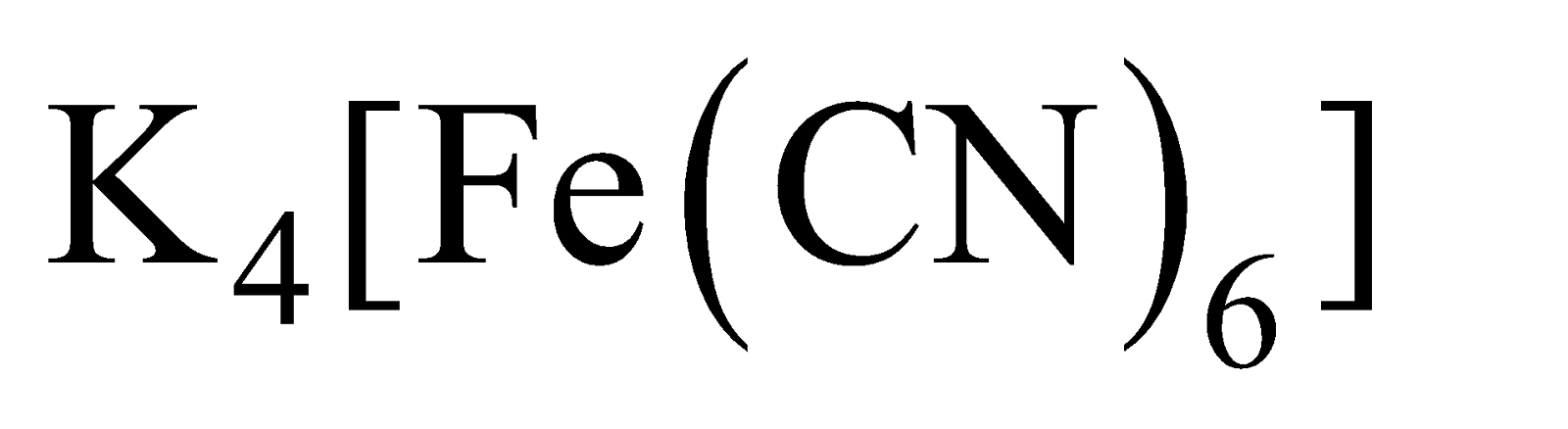 with
with 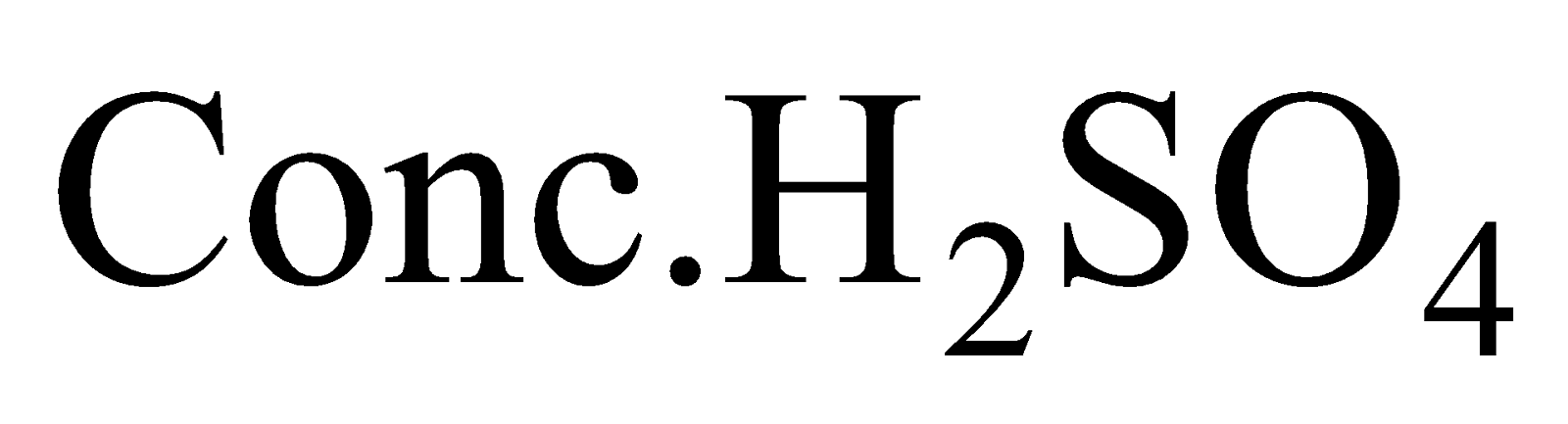
PROPERTIES
Neutral, colourless, poisonous, burns with blue flame. Sparingly soluble in water. With haemoglobin it gives “carboxy haemoglobin” which destroys its capacity to supply oxygen to the body.
- Burning - Non supporter of combustion. Burns in air with blue flame.
Reduces ammoniacal AgNO3
Reduces Fehling-solution
- Reducing nature - Metal oxides are reduced to metals.
- Unsaturated nature - It gives addition products.
- Formation of metal carbonyls - It acts as lewis base.
USES
In metallurgy of Ni-Monds process
Manufacture of methanol, phosgene, synthetic petrol, as reducing agent.
TEST
- Burns with blue flame.
- Reduces iodine pentoxide to I2.
Carbogen - (mixture of 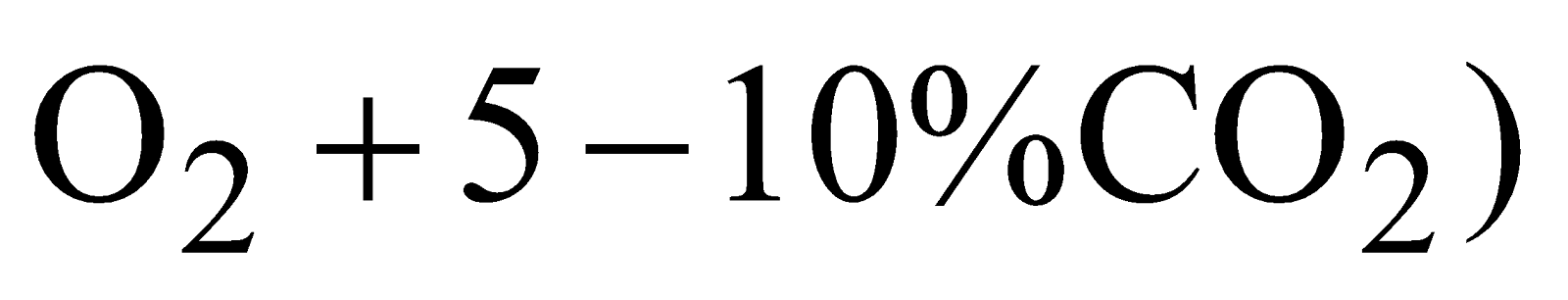 is used for artificial respiration for victims of CO poisoning.
is used for artificial respiration for victims of CO poisoning.
FUEL GASES
WATER GAS (H2 + CO)
Preparation : 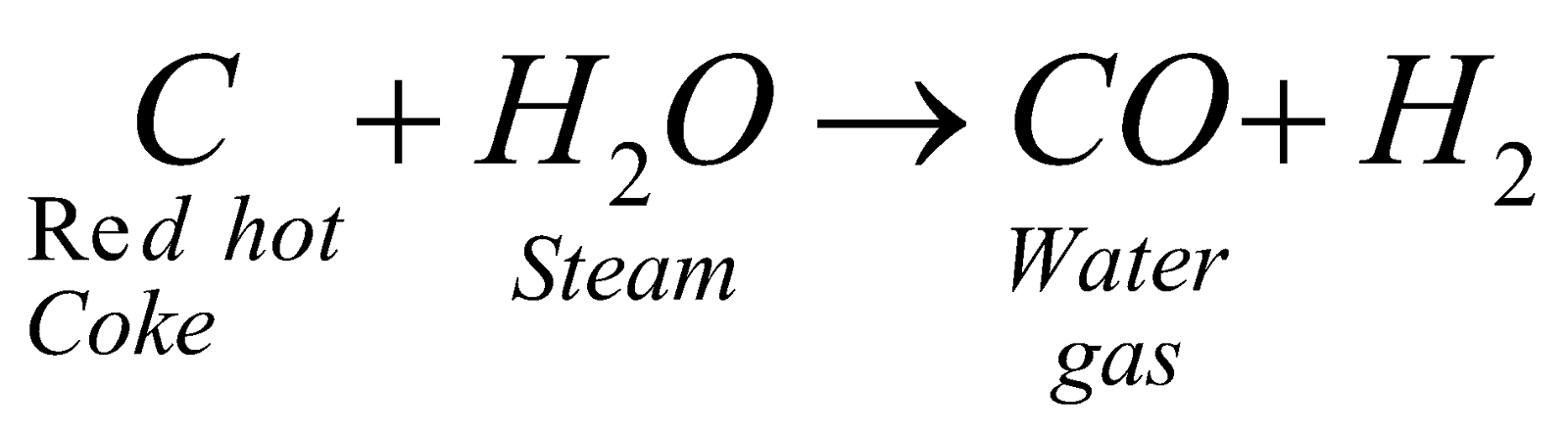 - 28 kcal
- 28 kcal
Uses : Burns with blue flame, calorific value 2700 kcal/m3. Industrial source of hydrogen (Bosch process). Manufacture of methyl alcohol (Patart Process) - Synthetic petrol (Fischer-Tropsch process). For making carburetted water gas.
SEMI WATER GAS
(mixture of water gas and producer gas)
Preparation : 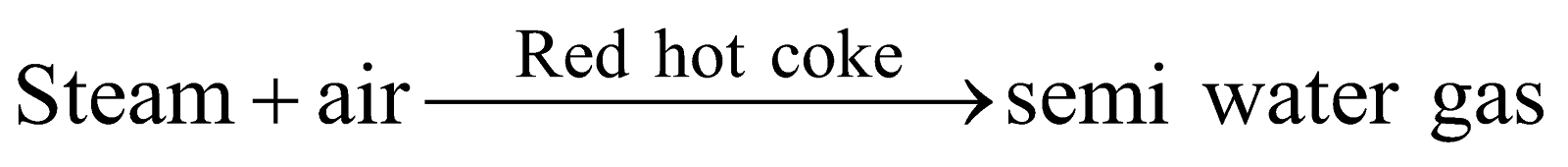
Composition : CO = 27.0%, H 2= 10.9%
CH4 = 1.28%, CO2 = 4.50%
N2 = 56.3%
Properties and Uses : Its calorific value 160 - 180 B.T.U. per cubic foot. As fuel in steel industry and for production of power in internal combustion engine.
PRODUCER GAS
Composition : CO = 31.7%, N2 = 65.7%, CO2 = 2.5%
Properties and Uses : Poisonous, combustible but non supporter of combustion, has low calorific value. Mainly employed as fuel.
COAL GAS
Preparation : By destructive distillation of coal
Composition : H2 = 45-55% N2 = 2-12%
CH4 = 25-35%, CO2 = 0-3%
CO = 4-11%, O2 = 1-1.5%
Ethylene, acetylene, benzene etc = 2.5-5%
Uses : Used as illuminant, as fuel, to provide inert atmosphere in metallurgical processes.
NATURAL GAS
It is found along with petroleum below the surface of earth.
Composition : CH4 = 60-80%
Higher hydrocarbons = 2-14%
C2H6 = 5-9%, C3H8 = 3-18%
Uses : It is used as a fuel. Its partial combustion yields carbon black (reinforcing agent for rubber)
OIL GAS
Preparation :
Uses : It is used as fuel in laboratories in Bunsen Burners.
WOOD GAS :
Preparation : 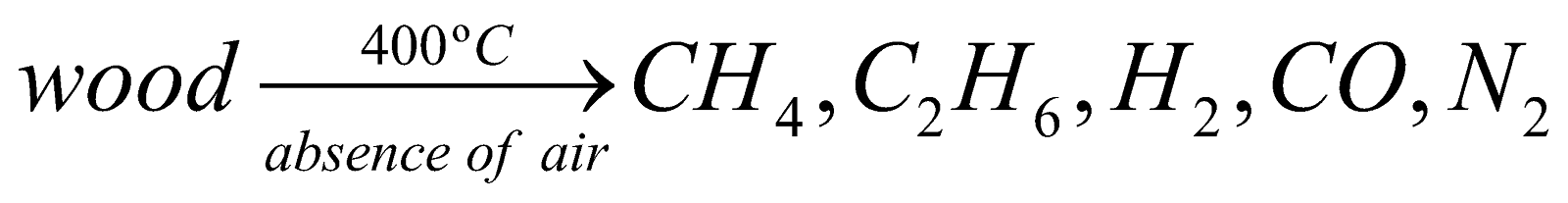
Uses : It is used as fuel
LIQUIFIED PETROLEUM GAS (LPG)
Composition : n-Butane + Iso-butane
Uses : It is used as domestic fuel.
GOBAR GAS :
Preparation :
Uses : As domestic fuel.
British Thermal Unit (B.T.U.) - It represents the amount of heat required to raise the temperature of one pound of water through 1ºF. One B.T.U. is equal to 252 calories.
SILICON
Silicon does not occur free in nature . In abundance it is next to .
OCCURRENCE
As oxide  (Silica )in sand, quartz, flint. As silicates
(Silica )in sand, quartz, flint. As silicates
of Al, Mg , K, Fe etc. Aluminium silicate is most
widely distributed as Felspar (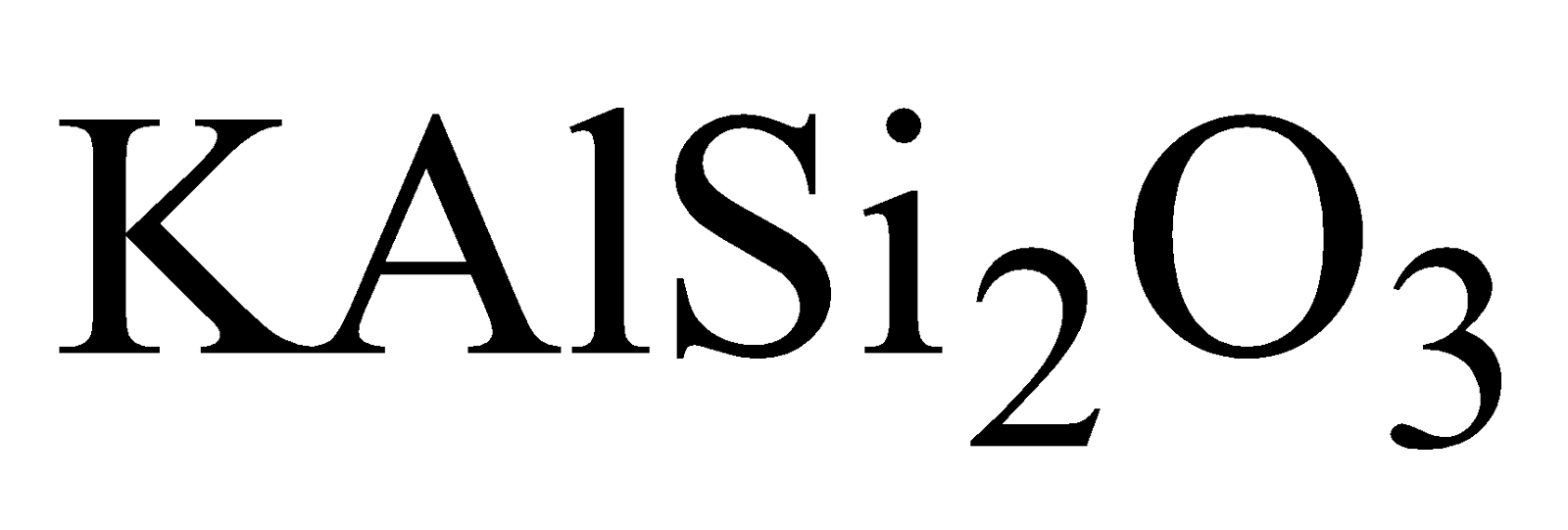 )and Mica
)and Mica 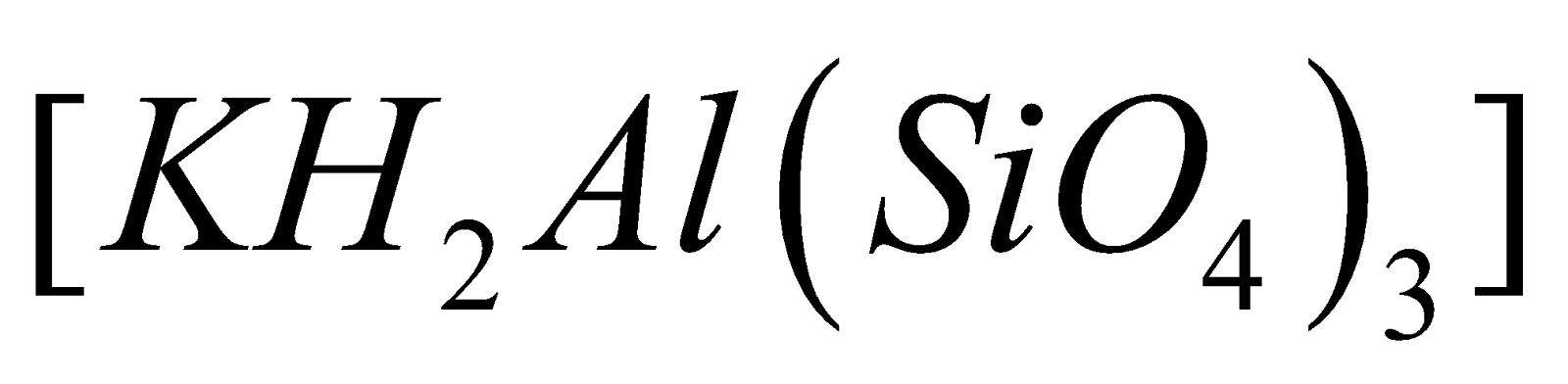 , Feldspar (
, Feldspar (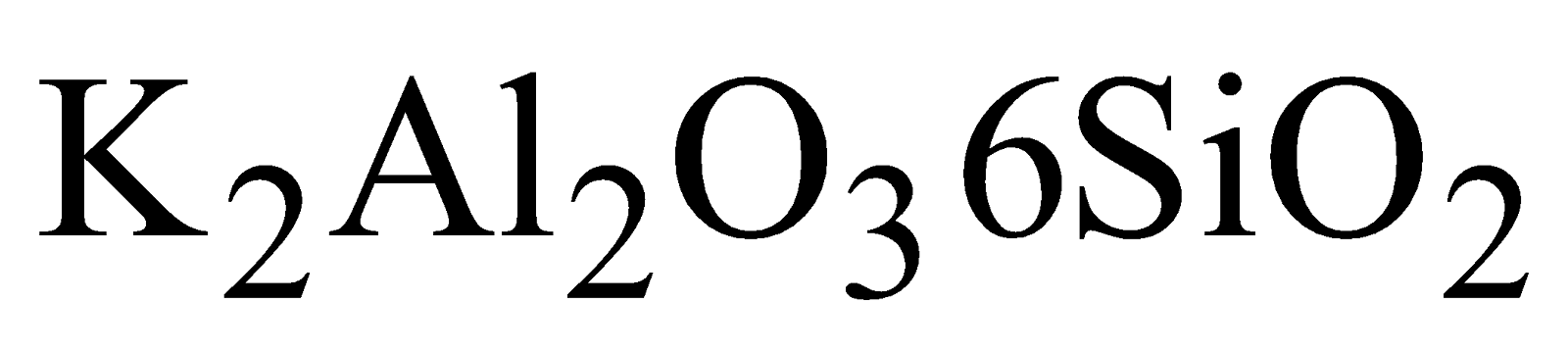 ), Kaolinite
), Kaolinite 
of Al, Mg , K, Fe etc. Aluminium silicate is most
widely distributed as Felspar (
PREPARATION OF AMORPHOUS FORM
It is very common and may be obtained by heating powdered quartz or finely divided silica with Mg powder.
PREPARATION OF CRYSTALLINE FORM
By reduction of 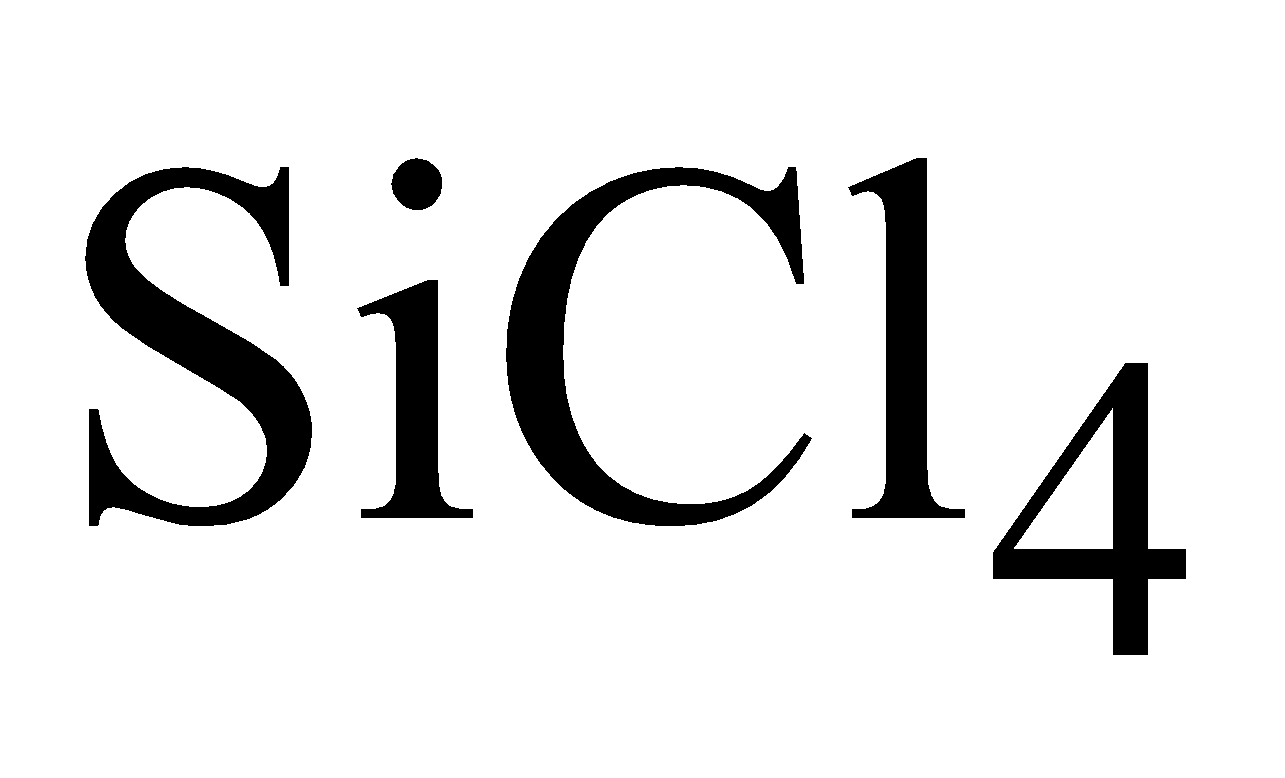 with molten Al
with molten Al
By reduction of highly purified SiCl4 with hydrogen
Zone-refining
Silicon is purified by Zone-refining process because the impurities present in it are more soluble in the liquid phase than in the solid phase.
PROPERTIES
Crystalline form possesses metallic lustre. It is very hard and scratches glass. Crystalline silicon is isomorphous with diamond.
Chemical reactions of amorphous silicon: Burns in air
USES
Silicon chips used for computing devices are doped with P,As, Al or Ga to enhance the semiconductor properties.
COMPOUNDS OF SILICON
- SiO2 (silica) - It exists in three crystalline forms Quartz, Tridymite and cristobalite. Further each form has
and
form. At low temperature the
form is stable and at high temperature the
form is stable .
- Sand - It is crushed form of quartz produced in nature by weathering of rocks .
- Flint - It is amorphous silica associated with quartz .
- Kieselguhr - Siliceous rock composed of the remains of sea organisms. Used as absorbent for nitroglycerine.
- Quartz or rock crystal - It is purest form of silica It is optically active.
- Silicic acid
Ortho silicic acid 
Metasilicic acid 
- Silicic acid sol - Colloidal solution of silicic acid
- Silica gel - Transparent gel of bluish white colour
- Water glass or sodium silicate - Sodium silicate containing excess of SiO2.
- Silica garden - Aqueous solution of sodium silicate containing crystals of various coloured salts e.g. copper sulphate, cobalt nitrate , manganese chloride, nickel chloride etc.
- Hydrofluorosilicic acid H2SiF6
- Permutit - Artificially prepared sodium aluminium silicate containing varying composition of sodium ,aluminium and silica
.
Used for softening hard water. - Zeolites -They have honeycomb like structure and have the general formula
They act as ion exchanger and molecular sieves. They can be artificially prepared by heating China clay, Silica and
- Ultramarines - Zeolite type silicates, containing ions like
and not water, are known as ultramarines e.g.
. Many of them are coloured and used as pigments and calico printing.
- Carborundum - silicon carbide
It is nearly as hard as diamond.
GLASS
Amorphous, hard, brittle, transparent, translucent supercooled solution of various silicates and borates of K, Ca and Pb. It has no definite formula but roughly can be represented as
Raw material used in the manufacture of glass.
- SiO2
- Na2CO3, K2CO3 or NaNO3, KNO3
- Alkaline earth metals e.g. CaCO3, BaCO3
- Oxides of heavy metals
- Cullets (pieces of glass)
- Colouring matter
MANUFACTURE
Mixture of raw materials  till CO2 escapes
till CO2 escapes  clear liquid. After some cooling it is used for casting articles
clear liquid. After some cooling it is used for casting articles
Annealing - Process of slow cooling of glass is known as annealing. The glass becomes soft.
TIN (Sn)
PRINCIPAL ORES OF TIN
- Cassiterite or Tin stone, SnO2
- Tin Pyrites, SnS.Cu2S.FeS.
EXTRACTION
- Concentration - By gravity process washing with water and then magnetic separation.
- Roasting -To remove volatile impurities
- Smelting - It is carried out in reverberatory furnace with coal (powdered anthracite) and limestone.
Sn so obtained contains iron and other metals and called black tin .
- Refining by (a) Liquation (b) Poling and (c) Electrolytic
PROPERTIES
Soft silvery white metal, ductile and malleable. It has maximum number of isotopes and three allotropic forms.
Grey⇌ 18℃ White ⇌161℃ Rhombic
Tin cry - It produces a peculiar cracking sound on bending which is known as tin cry.
Tin plague - It is the conversion of white tin to grey tin at low temperature which crumbles into powder.
Tinning - Since tin is not attacked by organic acids the utensils are protected by thin layer of tin .A pinch of is sprinkled over hot and clean surface, when HCl liberated removes the oxide film.Tin then rubbed over the clean surface with the help of rag dipped in
is sprinkled over hot and clean surface, when HCl liberated removes the oxide film.Tin then rubbed over the clean surface with the help of rag dipped in  powder. The utensil is immediately dipped in water to avoid oxide formation .
powder. The utensil is immediately dipped in water to avoid oxide formation .
Tin plating - It involves the deposition of thin protective layer of tin over sheets of iron electrolytically.
CHEMICAL PROPERTIES
With acids
Metastannic acid
COMPOUNDS OF TIN
STANNIC OXIDE (SnO2)
It occurs naturally as cassiterite.
PREPARATION
PROPERTIES
White solid, insoluble in water and amphoteric in nature.
USES
As polishing powder, in glass and pottery manufacture.
STANNOUS OXIDE (SnO)
PREPARATION
PROPERTIES
It is black solid and amphoteric in nature.
SULPHIDES
SnS precipitated by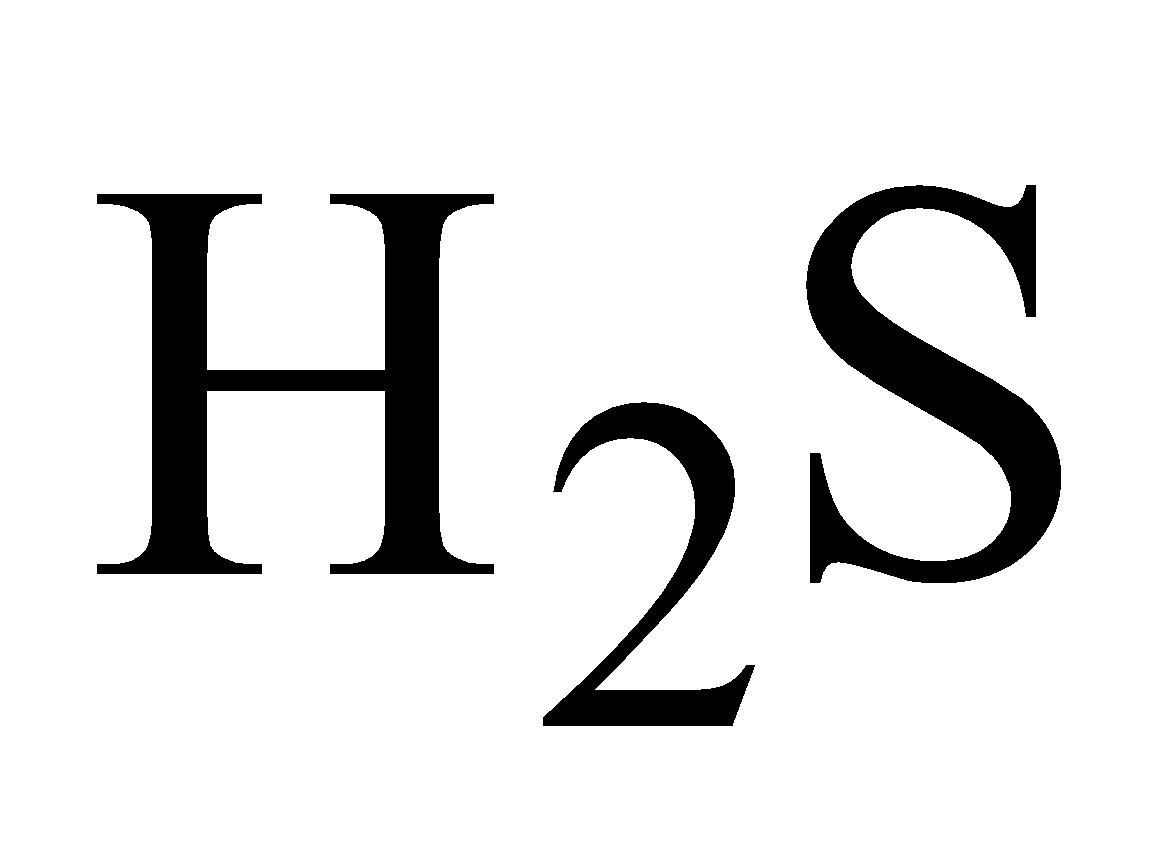 .It is dark brown solid, soluble in yellow ammonium sulphide forming
.It is dark brown solid, soluble in yellow ammonium sulphide forming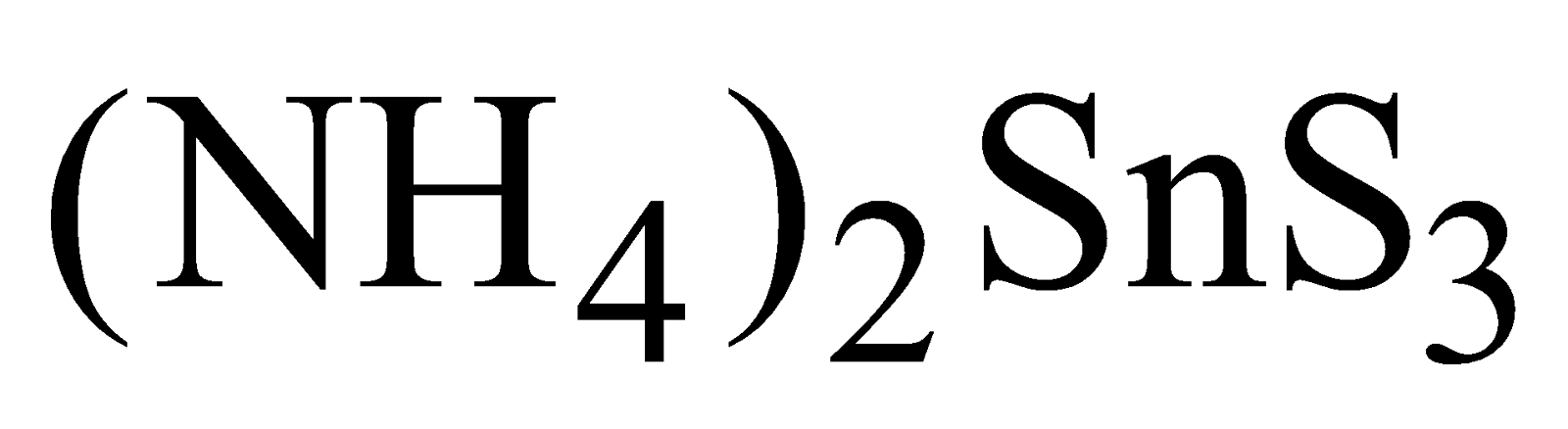
STANNOUS CHLORIDES (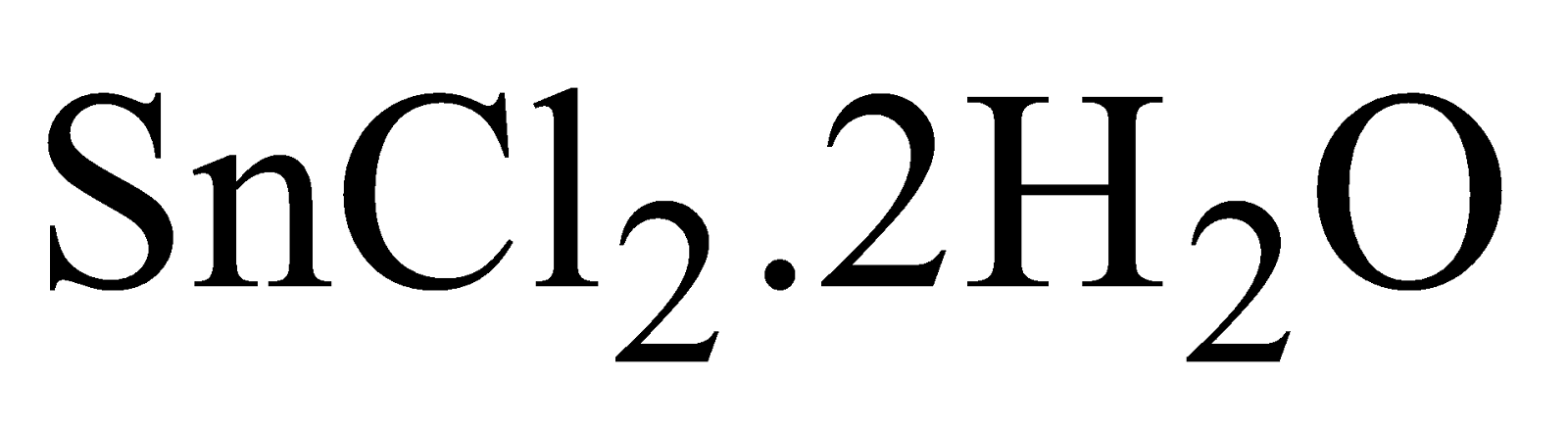 )
)
PREPARATION
Anhydrous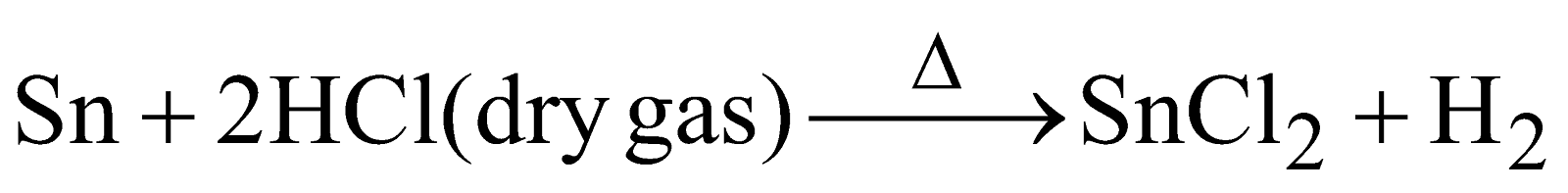
PROPERTIES
- White crystalline solid, soluble in water, alcohol and ether.
- Hydrolysed in water
- Strong reducing agent
BUTTER OF TIN
used as mordant in dyeing.
PURPLE OF CASSIUS
Colloidal particles of gold absorbed by stannic acid Sn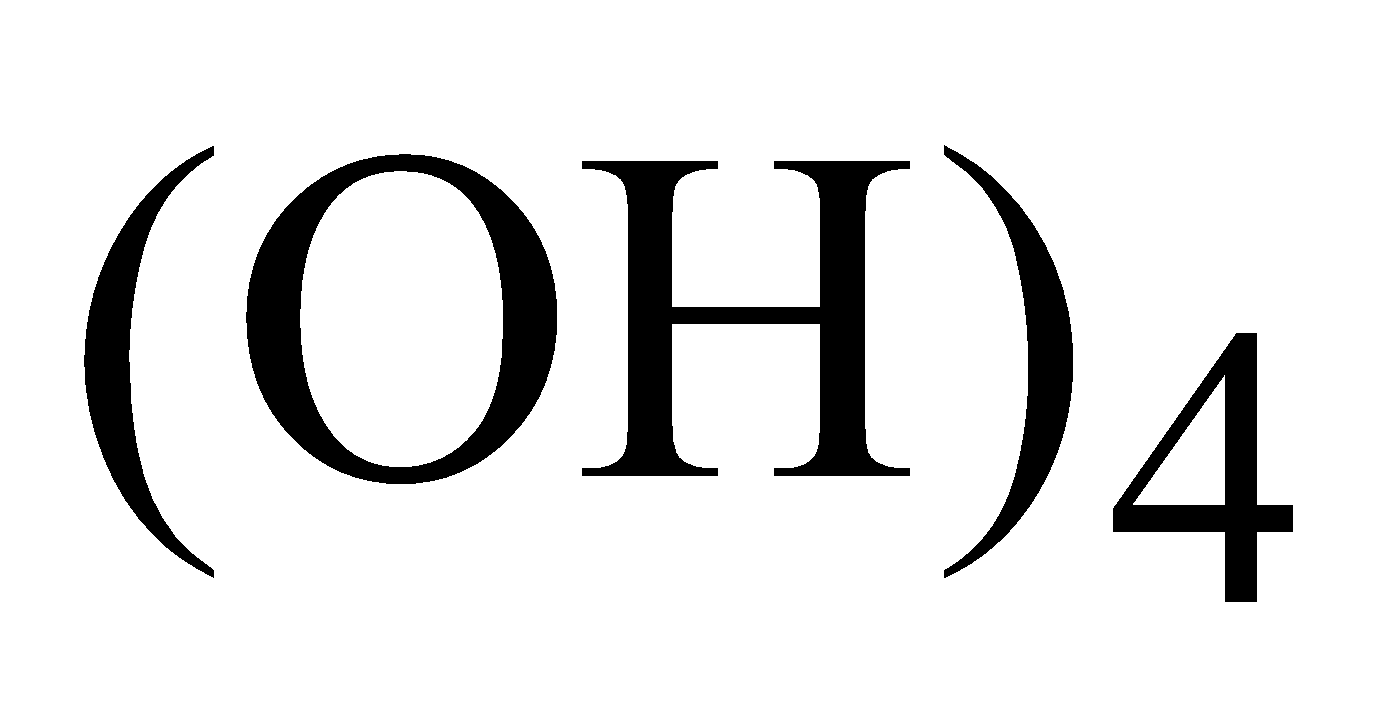 is known as purple of cassius. It is used for colouring glass and pottery.
is known as purple of cassius. It is used for colouring glass and pottery.
ALLOYS OF TIN
Some important alloys of tin are
- Solder Sn 50-70% Pb 30-50%
- White metal Sn 82% Sb 12% Cu 6%
- Britannia metal Sn 90% Sb 7% Cu 3%
- Soluminium Sn 55% Zn 33% Al 11% Cu 1%
- Babbitt metal Sn 90% Sb 7% Cu 2%
- Pewter Sn 80% Pb 20%
- Dental alloy Alloy of Sn, Ag, and Hg
LEAD
PRINCIPAL ORES
- Galena PbS
- Anglesite PbSO4
- Cerussite PbCO3
EXTRACTION
From galena - Two important processes are:-
- Air reduction process
(i) Concentration - By froth floatation process
(ii) Roasting - Roasted in air at 500°C – 600°C.
- Carbon reduction process
Mixed sulphides (PbS & ZnS) are roasted to obtain oxides which are fed into blast furnace with coke and lime.
Molten lead tapped off from the bottom.
PURIFICATION
Electrolytic method to remove Cu, Ag, Au, Sb etc.
PROPERTIES
Bluish, grey lustrous metal which acquires dull appearance when exposed to air due to formation of basic carbonate (Pb(OH)2.PbCO)3. Poor conductor of electricity.
CHEMICAL PROPERTIES
Plumbo Solvency - Formation of lead hydroxide with  in presence of air.
in presence of air.
Hence lead is readily corroded hard water has no action on lead which forms a protective layer of  and
and 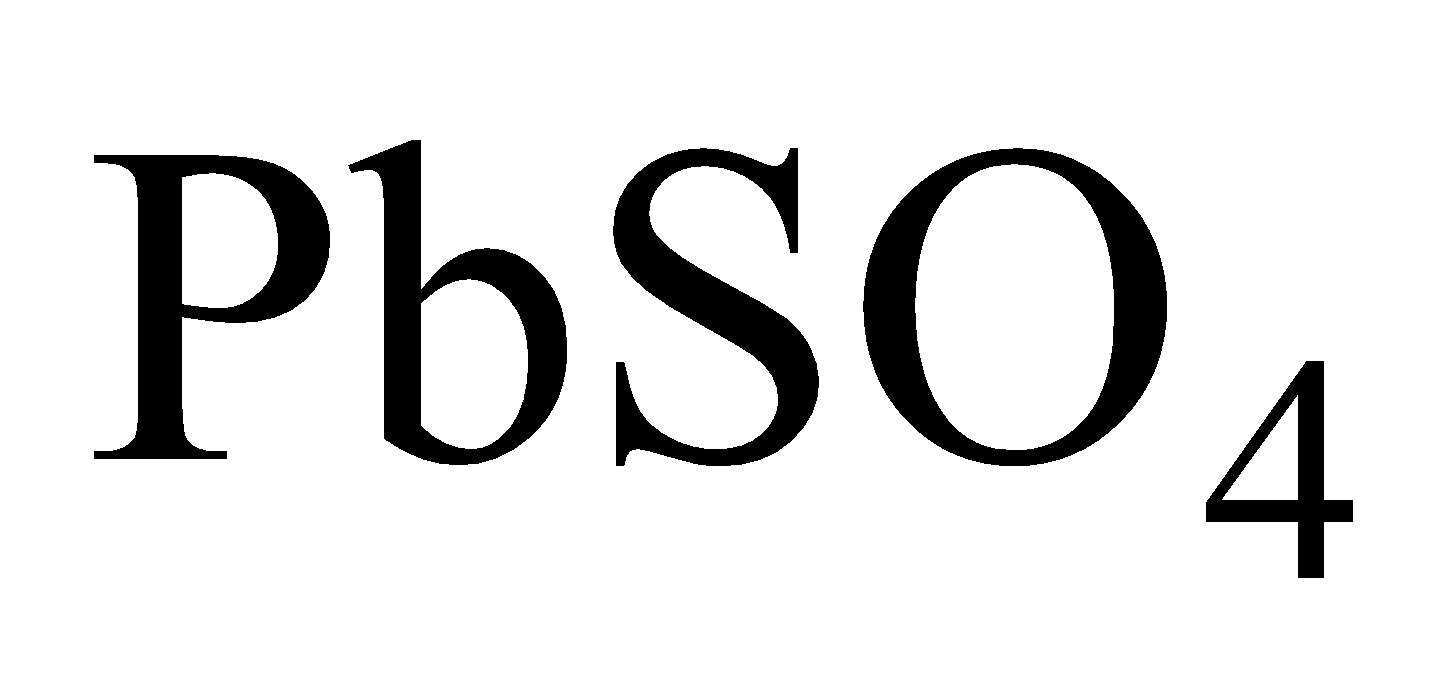
Hence hard water can be conveyed through lead pipes.
USES
In lead accumulators, pigments such as red lead, chrome yellow, chrome red, (C2H5)4Pb as antiknock compound, manufacturing of sulphuric acid.
COMPOUNDS OF LEAD
LEAD MONOXIDE (PbO)
PREPARATION
PROPERTIES
Insoluble in water and amphoteric in nature.
USES
Making glass, pottery, Massicot mixed with glycerine joins glass and stone . As drier in paints and varnishes.
LEAD DIOXIDE ( )
)
PREPARATION
By any of the methods given below
PROPERTIES
It is brown solid. Powerful oxidising in nature.
Amphoteric in nature
USES
Storage batteries and match industry, as oxidizing agent.
TRILEAD TETRAOXIDE, RED LEAD, MINIUM OR SINDHUR
PREPARATION
PROPERTIES
Red crystalline solid, insoluble in water.
Action of heat 
With acids
Structure
USES
In glass, match and pottery and as red pigment.
BASIC LEAD CARBONATE, WHITE LEAD [2PbCO3. Pb(OH)2]
PREPARATION
Dutch process - It is formed by exposing thin sheets of lead to vapours of acetic acid in presence of horse dung or tan bark .
PROPERTIES
White crystalline solid, turns black when exposed to due 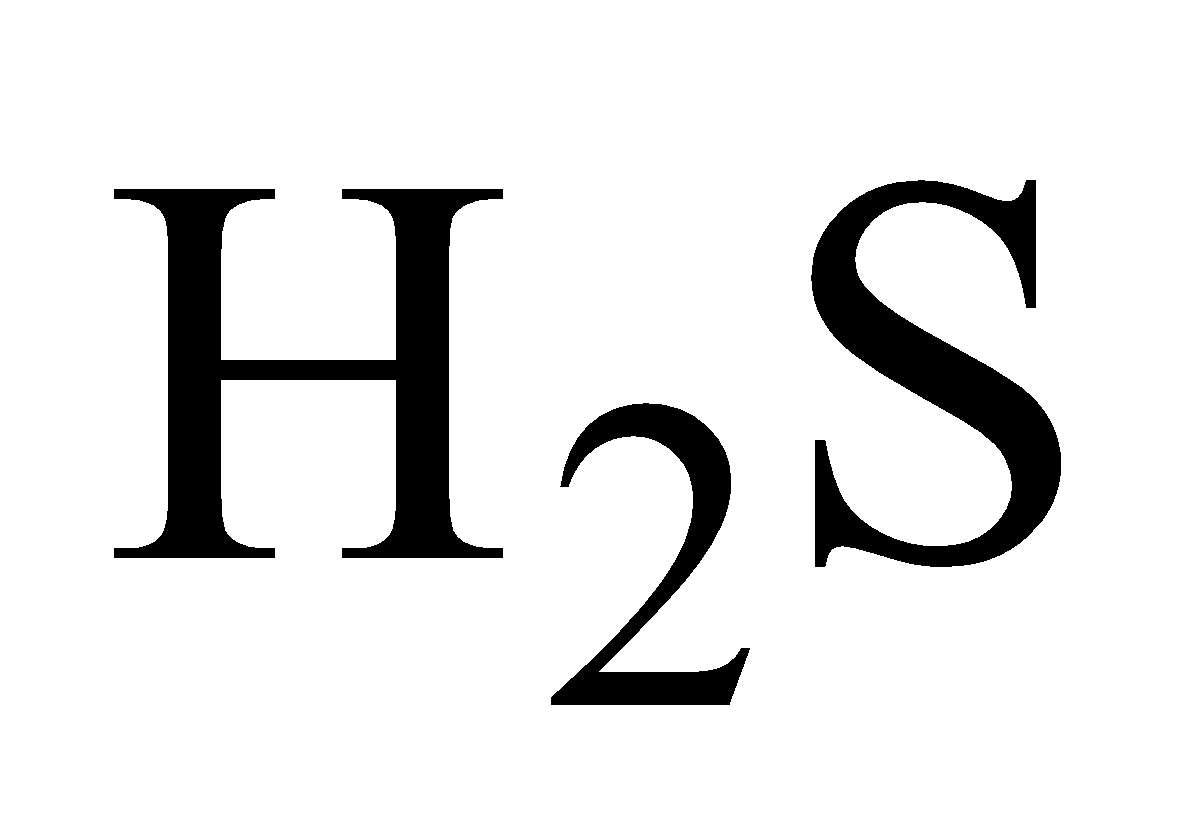 to PbS formation .It is highly poisonous.
to PbS formation .It is highly poisonous.
Action of heat
USES
As a white paint.
Halides of lead [Pb(II) halide]
PREPARATION
PROPERTIES
Covalent character
Pb (IV) halides :
PREPARATION
PROPERTIES
It is covalent liquid and unstable.
Sugar of lead -It has sweet taste.
-It has sweet taste.
Chrome yellow or Lemon chrome 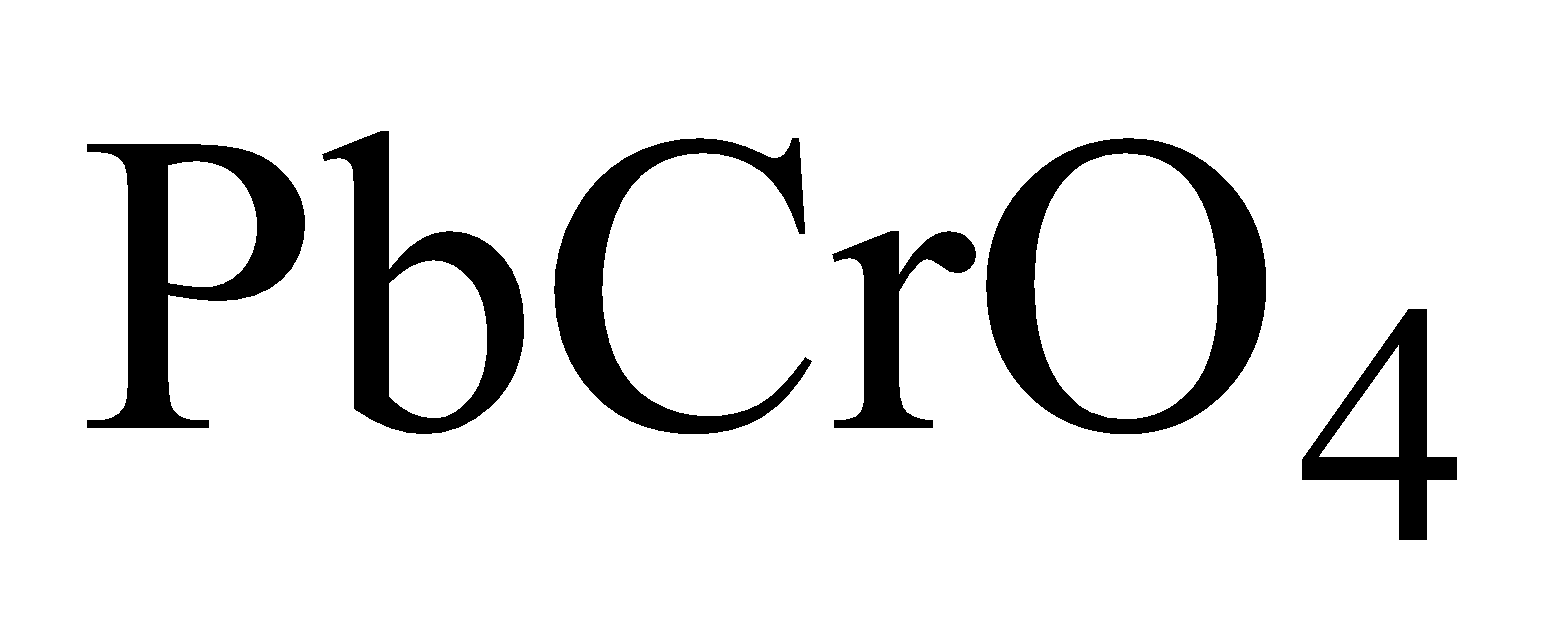
Basic lead Chromate or chrome red
Lead tetra ethyl
Antiknock agent.
Lithophone  is substitute of white lead.
is substitute of white lead.
FUSIBLE ALLOYS OF LEAD
- Type metal Pb 82% Sb 15% Sn 3 %
- Wood metal Bi 50% Pb 25% Sn 12.5% Cd 12.5%
- Lipowitz Bi 50% Pb 27% Sn 13% Cd 10%
- Rose metal Bi 50% Pb 28% Sn 22%
- Newton’s metal Bi 50% Pb 31% Sn 19 %










