PRINCIPLES RELATED TO PRACTICAL CHEMISTRY (PART-2)
CHEMICAL PRINCIPLES INVOLVED IN THE QUALITATIVE SALT ANALYSIS
A salt is a substance which is formed by the combination of an acid and base e.g
A salt thus consists of cation known as basic radical and anion known as acid radical. The identification of salts is known as inorganic analysis. The tests employed are
- Dry tests
- Wet tests
DRY TESTS
PHYSICAL APPEARANCE
It gives valuable information about certain salts eg
ACTION OF HEAT
Some salts change their colour when heated.
NaCl, KI, Pb(NO3)2 and Ba(NO3)2 – Decrepitate (Cracking sound)
Alums, bortates and phosphate – Swell up on heating
HgCl2, Hg2Cl2, Sb2O3, AS2O3, AlCl3 and NH4X – are white and sublime on heating
AS2O3, HgI2 – Yellow and sublime
GAS EVOLVED ON HEATING
FLAME TESTS
Salt + 1–2 drops of Conc HCl  Heat in a non luminous (oxidising) Bunsen flame using platinum wire.
Heat in a non luminous (oxidising) Bunsen flame using platinum wire.
BORAX BEAD TEST
Borax is heated on a loop of Pt. wire when colourless glassy bead of sodium metaborate and boric anhydride is formed
Na2B4O7 . 10H2O  Na2B4O7 2NaBO2 +B2O3
Na2B4O7 2NaBO2 +B2O3
Coloured salts are then heated on the glassy bead when coloured metaborate is formed in the oxidising flame
CuSO4+B2O3 Cu(BO2)2 +SO3
Cu(BO2)2 +SO3
Copper metaborate (blue)
In reducing flame we have 2Cu (BO2)2+ C 2CuBO2+B2O3+CO
2CuBO2+B2O3+CO
2Cu (BO2)2+2C 2Cu+2B2O3+2CO
2Cu+2B2O3+2CO
Hence different color appear in different flames
Note - Metals undergoing change in oxidation state eg Fe2+ and Fe3+ and Cu+ and Cu2+ form two metaborates
Fe2(SO4)3+2B2O3  2Fe(BO2)3 + 3SO3
2Fe(BO2)3 + 3SO3
Yellow (oxidising flame)
2Fe(BO2)3+C  2Fe(BO2)2 + B2O3 + CO
2Fe(BO2)2 + B2O3 + CO
Green (reducing flame)
CHARCOAL CAVITY TEST
Inorganic substance +(K2CO3& Na2CO3) fusion mixture in a charcoal cavity + Reducing flame with blowpipe. Observe the colour of bead formed or metal
CuSO4 +Na2CO3  CuCO3 + Na2SO4
CuCO3 + Na2SO4
CuCO3  CuO + CO2
CuO + CO2
CuO +C  Cu + CO
Cu + CO
Pb(NO3)2 +Na2CO3  PbCO3 + 2NaNO3
PbCO3 + 2NaNO3
PbCO3 PbO + CO2
PbO + CO2
PbO +C  Pb + CO
Pb + CO
COBALT NITRATE TEST
It is an extension of charcoal cavity test. If the residue left in charcoal cavity is white add one drop of cobalt nitrate solution and heat again in the oxidising flame and observe the Colour
Reactions
ZnSO4+Na2CO3 ZnCO3+Na2SO4
ZnCO3+Na2SO4
ZnCO3  ZnO+CO2
ZnO+CO2
ZnO+CoO  CoO.ZnO (Cobalt zincate)
CoO.ZnO (Cobalt zincate)
MgO+CoO CoO.MgO
CoO.MgO
Al2O3+CoO CoO.Al2O3
CoO.Al2O3
2Co(NO3)2 2CoO+4NO2+O2
2CoO+4NO2+O2
MICROCOSMIC BEAD TEST
It is similar to borax bead test. Here microcosmic salt Na(NH4)HPO4 is used in place of borax which forms a colourless, transparent bead on a loop of Pt. wire of sodium metaphosphate on heating
[Na(NH4)HPO4  NaPO3+NH3+H2O]
NaPO3+NH3+H2O]
The metallic oxides then combine with sodium metaphosphate to form coloured ortho phosphates eg
NaPO3+CoO 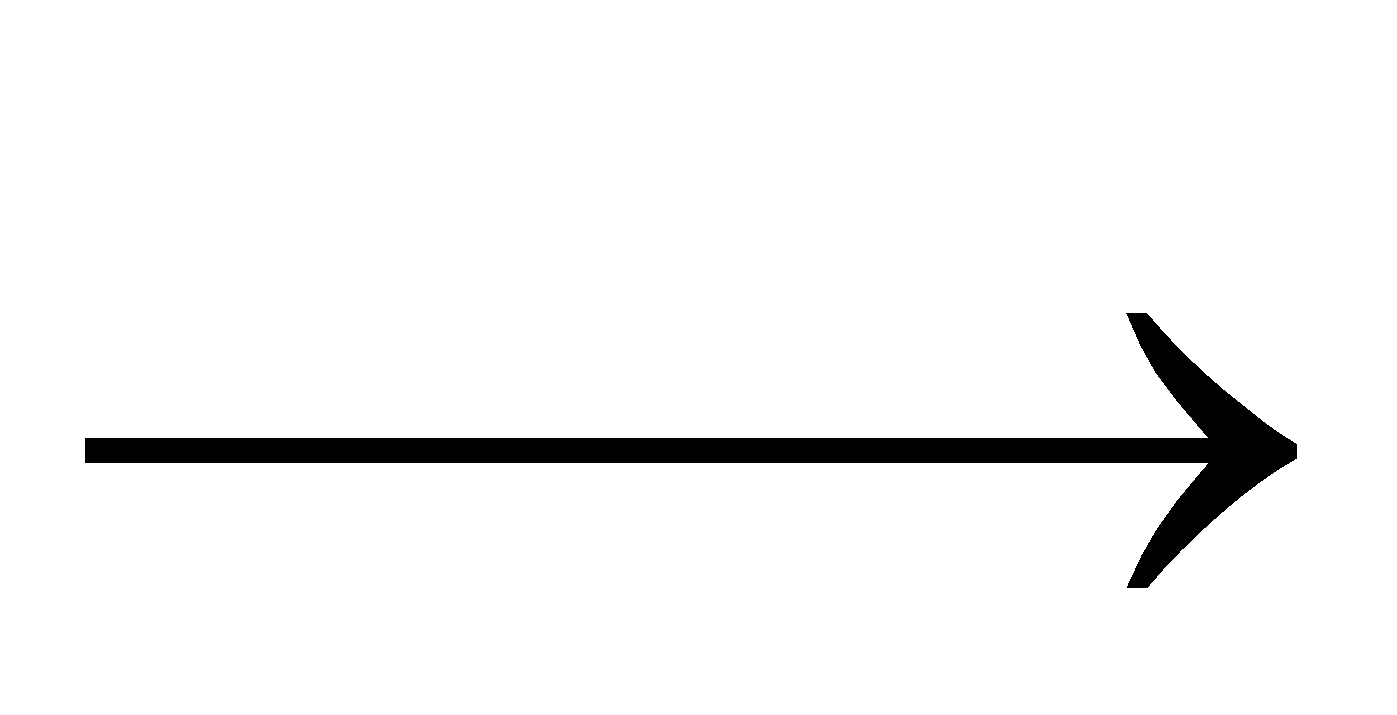 NaCo.PO4
NaCo.PO4
Cobalt orthophosphate blue
WET TESTS
IDENTIFICATION OF ACID RADICALS - TYPE I
The radicals decomposed by dil. H2SO4 or dil. HCl
IDENTIFICATION OF ACID RADICALS - TYPE II
The radicals decomposed by Conc. H2SO4
DETECTION OF ACID RADICALS - TYPE I
CARBONATE AND BICARBONATE 
Ca(OH)2+CO2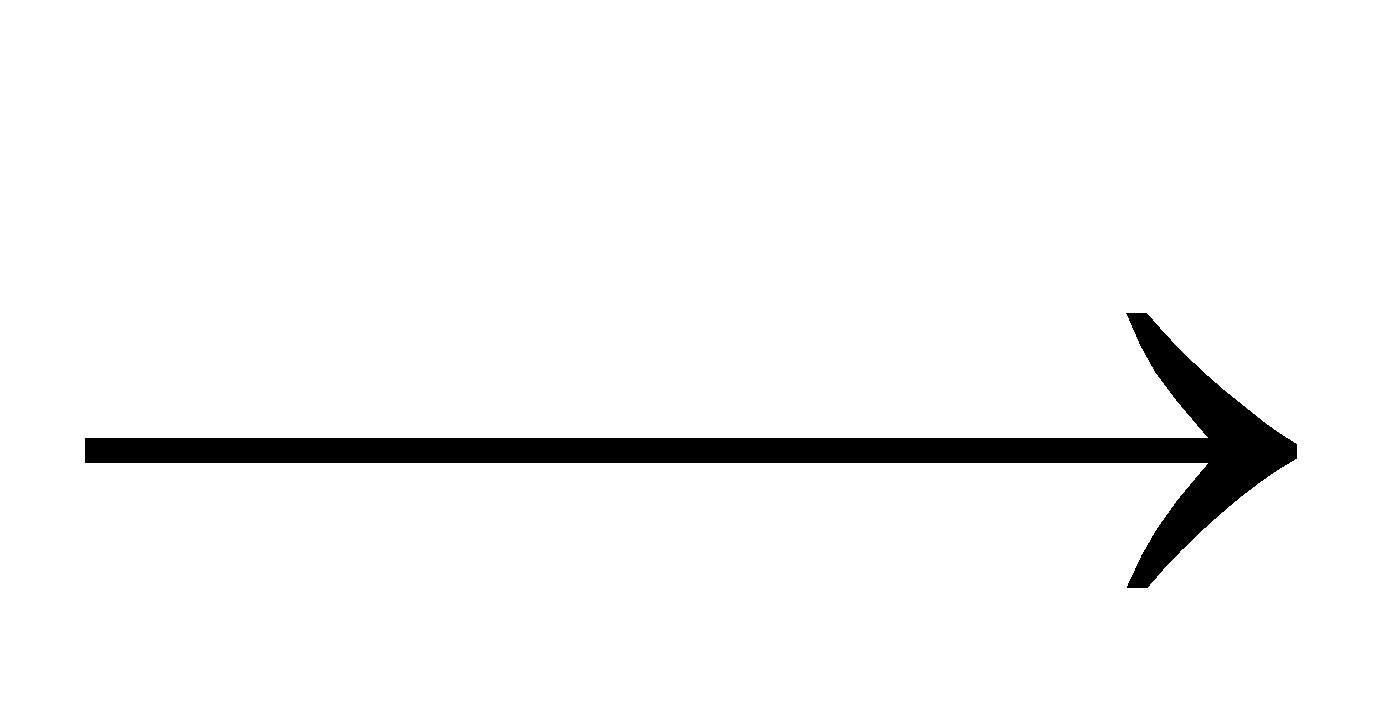 CaCO3+ H2O (white ppt)
CaCO3+ H2O (white ppt)
SO2 from sulphite also turns lime water milky but it has smell of burning sulphur.
SULPHITE 
SO2 gives white precipitate of BaSO3 with BaCl2 which is soluble in dil. HCl
SULPHIDE (S2–)
FeS+H2SO4  FeSO4+H2S
FeSO4+H2S
H2S+(CH3COO)2Pb  2CH3COOH+PbS (black)
2CH3COOH+PbS (black)
CdCO3+H2S 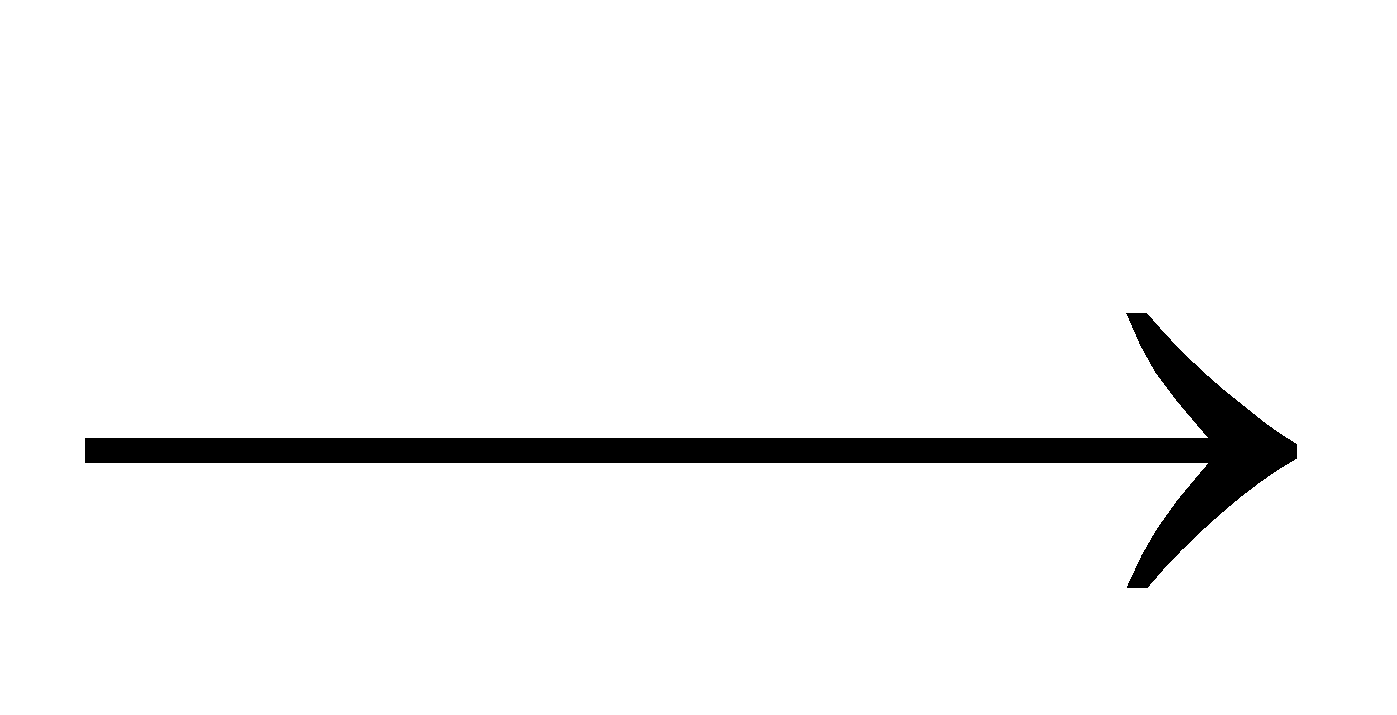 H2O+CO2+CdS (yellow)
H2O+CO2+CdS (yellow)
Sodium carbonate extract of inorganic mixture containing give violet colour with sodium nitroprusside
Na2S+Na2[Fe(CN)5NO]  Na4[Fe(CN)5NOS] Sodium thio nitroprusside
Na4[Fe(CN)5NOS] Sodium thio nitroprusside
Sulphides of Pb, Ca, Ni, Co, Sb and Sn (iv) are not decomposed by dil acid. Conc. HCl is used for them.
NITRITE
Ba(NO3)2 +H2SO4  BaSO4+2HNO2
BaSO4+2HNO2
2 HNO2  H2O + NO + NO2
H2O + NO + NO2
2 NO + O2  2 NO2
2 NO2
2 KI + 2NO2  2 KNO2 + I2
2 KNO2 + I2
Starch + I2 starch iodide blue
starch iodide blue
FeSO4.7H2O + NO  [Fe(H2O)5 NO] SO4 + 2 H2O
[Fe(H2O)5 NO] SO4 + 2 H2O
Brown colour complex
ACETATE
Ca + H2SO4  CaSO4+2CH3COOH
CaSO4+2CH3COOH
All acetates dissolve in water. Their aqueous solution with neutral FeCl3 give blood red colour.
blood red ferric acetate
DETECTION OF ACID RADICALS - TYPE II
CHLORIDE (Cl–)
NaCl + H2SO4  NaHSO4 + HCl
NaHSO4 + HCl
NH4OH + HCl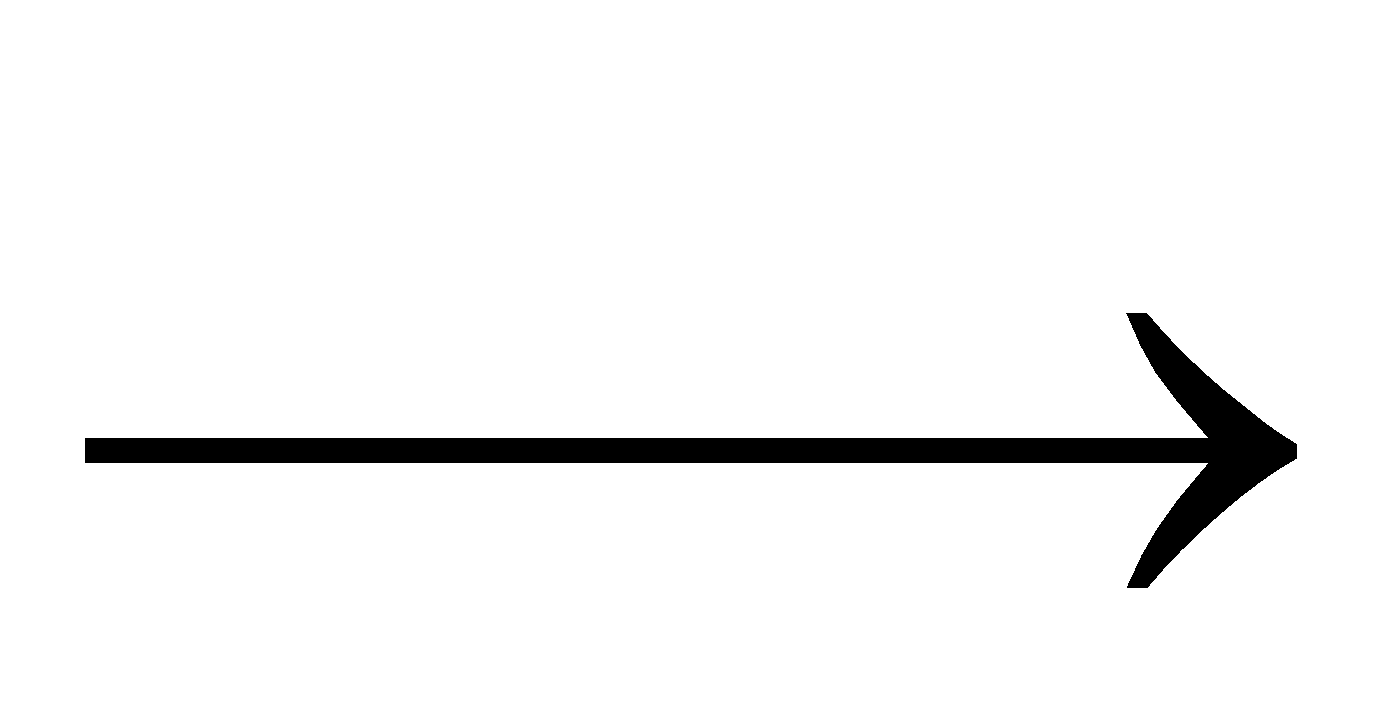 NH4Cl + H2O (white fumes)
NH4Cl + H2O (white fumes)
AgNO3 + HCl AgCl ¯ + HNO3 (white ppt)
AgCl ¯ + HNO3 (white ppt)
AgCl + 2NH4OH  [Ag(NH3)2]Cl Soluble complex + 2H2O
[Ag(NH3)2]Cl Soluble complex + 2H2O
Chromyl chloride test
4 NaCl + K2Cr2O7 + 3H2SO4  K2SO4 + 2 Na2SO4 + 2 CrO2Cl2 + 3H2O (Orange red)
K2SO4 + 2 Na2SO4 + 2 CrO2Cl2 + 3H2O (Orange red)
4 NaOH + CrO2Cl2  2 NaCl + Na2 CrO4+ 2H2O (Yellow solution)
2 NaCl + Na2 CrO4+ 2H2O (Yellow solution)
Na2CrO4 + (CH3COO)2 Pb 2 CH3COONa + PbCrO4 (Yellow precipitate)
2 CH3COONa + PbCrO4 (Yellow precipitate)
The test not is given by chlorides of Hg(ic), Sn, Ag, Pb, and Sb) and not by Br– and I–
BROMIDE (Br–)
2 KBr + H2SO4  K2SO4+ 2 HBr
K2SO4+ 2 HBr
2 HBr + H2SO4 2 H2O + SO2 + Br2
2 H2O + SO2 + Br2
More brown fumes when MnO2 is added
2NaBr + MnO2 + 3H2SO4  2 NaHSO4 + MnSO4 + 2H2O + Br2
2 NaHSO4 + MnSO4 + 2H2O + Br2
NaBr + AgNO3  AgBr ¯ + NaNO3 (yellow ppt)
AgBr ¯ + NaNO3 (yellow ppt)
AgBr + 2NH4OH  [Ag(NH3)2]Br + 2 H2O
[Ag(NH3)2]Br + 2 H2O
2NaBr + Cl2  2 NaCl + Br2 Soluble complex
2 NaCl + Br2 Soluble complex
Br2 + CHCl3 brown layer
brown layer
Excess of chlorine water should be avoided since layer becomes colourless due to conversion of Br2 into HBrO
Br2 + 2H2O +Cl2  2 HBrO + 2 HCl
2 HBrO + 2 HCl
IODIDE (I–)
KI + H2SO4  KHSO4+ HI
KHSO4+ HI
H2SO4 + 2HI  H2O + I2 + SO2
H2O + I2 + SO2
I2 + starch blue colour
2KI + MnO2 + 3H2SO4  2KHSO4 + MnSO4 + 2H2O + I2 (more fumes)
2KHSO4 + MnSO4 + 2H2O + I2 (more fumes)
NaI + AgNO3  AgI + NaNO3 (yellow ppt)
AgI + NaNO3 (yellow ppt)
AgI insoluble in NH4OH as it does not form any complex
2NaI + Cl2 2NaCl + I2
2NaCl + I2
I2 + CHCl3  Violet
Violet
I2 + 5Cl2 (excess)+ 6H2O  2HIO3 + 10 HCl violet colour disappear
2HIO3 + 10 HCl violet colour disappear
NITRATE
Mg(NO3)2 + H2SO4 MgSO4 + 2HNO3
MgSO4 + 2HNO3
4HNO3  2H2O +4NO2 + O2 (brown)
2H2O +4NO2 + O2 (brown)
On adding copper turnings the brown fumes intensify
Cu + 4HNO3  Cu(NO3)2 + 2NO2 + 2H2O
Cu(NO3)2 + 2NO2 + 2H2O
Ring test - 2FeSO4 + NO  FeSO4NO brown ring or
FeSO4NO brown ring or
[Fe(H2O)6]2+ + NO  [Fe(H2O)5NO]2+ + H2O
[Fe(H2O)5NO]2+ + H2O
FLUORIDE (F–)
Sodium carbonate extract after neutralisation with acetic acid is treated with CaCl2, a white precipitate insoluble in dil H2SO4 indicates the presence of F–
2NaF + CaCl2  2NaCl +CaF2 (White ppt)
2NaCl +CaF2 (White ppt)
CaF2 + Conc H2SO4 CaSO4 + 2HF
CaSO4 + 2HF
SiO2 + 4HF  SiF4 + 2H2O
SiF4 + 2H2O
3SiF4 + 3H2O  H2SiO3 + 2H2SiF6
H2SiO3 + 2H2SiF6
Silicic acid Hydrofluorosilicic acid
(Waxy white deposit)
OXALATE
Na2C2O4 + H2SO4  Na2SO4 + H2O + CO + CO2
Na2SO4 + H2O + CO + CO2
2CO + O2  2CO2
2CO2
CO burns with blue flame
Na2CO3 extract + excess CH3 COOH (neutralise) + CaCl2  White ppt
White ppt
soluble in dil H2SO4
soluble in dil H2SO4
Na2C2O4+ CaCl2 2NaCl +CaC2O4 (white ppt)
2NaCl +CaC2O4 (white ppt)
CaC2O4 + dil H2SO4 CaSO4 + H2C2O4
CaSO4 + H2C2O4
acidified solution of white ppt. decolourises KMnO4 solution
2KMnO4 + 3H2SO4 + 5H2C2O4 K2SO4 + 2MnSO4 + 8H2O + 10CO2
K2SO4 + 2MnSO4 + 8H2O + 10CO2
SULPHATE
Na2SO4 + BaCl2 2NaCl + BaSO4 (white ppt.)
All sulphates except Ba, Sr, Ca and Pb are soluble in water
BORATE
2Na3BO3 + 3H2SO4 3 Na2SO4 + 2H3BO3
3 Na2SO4 + 2H3BO3
H3BO3 + 3C2H5OH (C2H5)3 BO3 +3 H2O
BO3 +3 H2O
ethyl borate
(burns with green flame)
PHOSPHATE (PO43-)
Phosphates give canary yellow precipitate with ammonium molybdate
Phosphates give canary yellow precipitate with ammonium molybdate
Ca3(PO4)2 + 6HNO3  3Ca(NO3)2 + 2H3PO4
3Ca(NO3)2 + 2H3PO4
H3PO4 + 12(NH4)2MoO4 + 2lHNO3 (NH4)3PO4.12MoO3 + 21NH4NO3 + 12H2O
(NH4)3PO4.12MoO3 + 21NH4NO3 + 12H2O
Ammonium phosphomolybdate
(canary yellow ppt.)
(canary yellow ppt.)
SODIUM CARBONATE EXTRACT
One part inorganic compound or mixture + three parts of Na2CO3+25–30ml H2O. Boil the solution for 10–15 minutes and filter. The filtrate is known as sodium carbonate extract. By double decomposition insoluble carbonates of cations are formed and soluble sodium salts of anions
PbSO4 + Na2CO3  PbCO3
PbCO3  + Na2SO4
+ Na2SO4
CaCl2 + Na2CO3  CaCO3
CaCO3  + 2 NaCl
+ 2 NaCl
BaCl2 + Na2CO3  BaCO3
BaCO3  + 2NaCl
+ 2NaCl
Sodium Carbonate extract is basic in nature and before the test of anions it is neutralised with suitable acid.
Now basic radicals can be tested from I to IV groups without removing interfering radicals like phosphate, oxalate, fluoride and borate.
ANALYSIS OF BASIC RADICALS
Preparation of the original solution - The solution must be prepared in solvent following the order.
- Water
- dil HCl
- Conc. HCl
- dil HNO3
- Conc. HNO3
- Aqua regia
Conc HNO3 and Conc H2SO4 must be avoided for preparing the solution. Conc HNO3 precipitates and converts sulphides of Ba, Sr, Pb to insoluble sulphates.
- 2HNO3 + H2S
2H2O + 2 NO2 + S
Sulphuric acid also forms insoluble sulphates of Ba, Sr and Pb - If mixture is completely soluble in cold dil. HCl Ag+, Pb2+, are absent
- Hot Conc HCl solution on cooling or dilution give needle like crystals, Pb2+ is present and if turbidity appears Bi 3+or Sb3+ may be present
BiCl3 + H2O  BiOCl +2HCl
BiOCl +2HCl
The precipitate disappears if little HCl is added
- A white crystalline precipitate of NaCl and BaCl2 is formed by adding Conc HCl due to common ion effect
SEPARATION OF METALLIC IONS INTO GROUPS
DETECTION OF BASIC RADICALS - GROUP I
LEAD (Pb++)
Pb(NO3)2 + 2HCl  PbCl2 + 2HNO3 white ppt. soluble in hot H2O
PbCl2 + 2HNO3 white ppt. soluble in hot H2O
PbCl2 +HCl (excess)  H[PbCl3] soluble complex
H[PbCl3] soluble complex
- KI :
PbCl2 + 2KI  PbI2 + 2KCl
PbI2 + 2KCl
yellow ppt.
PbI2 + 2KI K2[PbI4] soluble complex decomposes on dilution
K2[PbI4] soluble complex decomposes on dilution
- dil H2SO4 :
PbCl2 + H2SO4PbSO4 + 2HCl
white ppt
PbSO4 + 2CH3COONH4 (CH3COO)2 Pb + (NH4)2SO4
(CH3COO)2 Pb + (NH4)2SO4
Soluble
- K2CrO4 :
PbCl2 + K2CrO4PbCrO4 + 2 KCl
yellow ppt
PbCrO4 + 4NaOH Na2PbO2 + Na2CrO4 + 2H2O
Na2PbO2 + Na2CrO4 + 2H2O
soluble
- H2S :
2PbCl2 +H2S Pb2SCl2 + 2HCl
Pb2SCl2 + 2HCl
Red ppt.
(Lead sulfochloride)
(Lead sulfochloride)
Pb2SCl2 + H2S  2PbS + 2HCl
2PbS + 2HCl
Black
3PbS +8HNO3  3Pb(NO3)2 + 2NO + 3S +4H2O
3Pb(NO3)2 + 2NO + 3S +4H2O
SILVER (Ag+)
- dil HCl :
AgNO3 + HClAgCl
+ HNO3
(white ppt)
AgCl +2NH4OH Ag[(NH3)2]Cl + 2H2O
Ag[(NH3)2]Cl + 2H2O
Soluble complex
- Dil HNO3 :
Ag(NH3)2Cl + 2HNO3AgCl
+
- KI :
Ag(NH3)2Cl + KIAgI + 2NH3 + KCl
yellow ppt
- K2CrO4 Solution :
2Ag (NH3)2Cl + K2CrO4Ag2CrO4 + 2KCl + 2 NH3
Brick red ppt.
MERCUROUS (Hg2++)
- dil HCl :
Hg2(NO3)2 + 2HCl  Hg2Cl2 + 2HNO3
Hg2Cl2 + 2HNO3
white ppt.
white ppt.
Hg2Cl2 + 2NH4OH  [Hg + Hg(NH2)Cl] + NH4Cl + 2H2O
[Hg + Hg(NH2)Cl] + NH4Cl + 2H2O
Black ppt soluble in aqua regia
HNO3 + 3HCl  NOCl + 2Cl + 2H2O
NOCl + 2Cl + 2H2O
Hg + 2Cl HgCl2
HgCl2
- SnCl2 solution :
2HgCl2 +SnCl2  Hg2Cl2 + SnCl4
Hg2Cl2 + SnCl4
white ppt
white ppt
Hg2Cl2 + SnCl2 2Hg + SnCl4
2Hg + SnCl4
Grey
Grey
- KI solution :
Hg2(NO3)2 + KI  Hg2I2 + 2KNO3
Hg2I2 + 2KNO3
green ppt
Hg2I2 + 2 KI  K2[HgI4] + Hg
K2[HgI4] + Hg
Black ppt (soluble)
- K2CrO4 soln. :
Hg2(NO3) + K2CrO4  Hg2CrO4 + 2KNO3
Hg2CrO4 + 2KNO3
Brown ppt.
DETECTION OF BASIC RADICALS - GROUP IIA
MERCURY (Hg)
- H2S :
3HgCl2 +2H2SHgCl2.2HgS + 4HCl (white)
HgCl2.2HgS + H2S 3HgS + 2 HCl (Black)
3HgS + 2 HCl (Black)
3HgS +
 3HgCl2 + 3S + 2 NO + 4 H2O
3HgCl2 + 3S + 2 NO + 4 H2O
- SnCl2 soln. :
2HgCl2 +SnCl2  Hg2Cl2 + SnCl4 white ppt
Hg2Cl2 + SnCl4 white ppt
Hg2Cl2+SnCl2 2Hg + SnCl4
2Hg + SnCl4
- KI Soln :
HgCl2 + 2KIHgI2 + 2KCl Red ppt
HgI2+ 2K I K2[HgI4] Soluble
I K2[HgI4] Soluble
- Copper turnings :
HgCl2 + Cu  CuCl2 + Hg grey film
CuCl2 + Hg grey film
BISMUTH (Bi)
- H2S :
2 BiCl3 + 3H2SBi2S3 + 6 HCl Black ppt
Bi2S3 + 8HNO3 2 Bi(NO3)3 + 2NO + 3S + 4H2O soluble
2 Bi(NO3)3 + 2NO + 3S + 4H2O soluble
- NH4OH :
Bi(NO3)3 + 3NH4OHBi(OH)3 + 3NH4NO3 white ppt
Bi(OH)3  BiO.OH + H2O yellow
BiO.OH + H2O yellow
Bi(OH)3 + 3 HCl BiCl3 + 2HCl (soluble)
BiCl3 + 2HCl (soluble)
- H2O :
BiCl3 + H2OBiOCl + 2HCl
white ppt
Bismuth oxychloride
Bi(NO3)3 + 2O  BiO(NO3) +2HNO3
BiO(NO3) +2HNO3
2Bi(NO3)3 + 3H2O  (BiO)2(OH)(NO3) + 5HNO3
(BiO)2(OH)(NO3) + 5HNO3
Basic salt
- Sodium stannite solution :
SnCl2 + 2NaOH  Sn(OH)2 + 2NaCl
Sn(OH)2 + 2NaCl
white ppt
Sn(OH)2 + 2NaOH  Na2SnO2 + 2H2O
Na2SnO2 + 2H2O
sod. stannite soluble
2BiCl3 + 3Na2 SnO2 + 6NaOH
SnO2 + 6NaOH
3H2O + 3Na2 SnO3 + 6NaCl + 2Bi (Black metallic)
SnO3 + 6NaCl + 2Bi (Black metallic)
- KI :
BiCl3 + 3KIBiI3 + 3KCl
Brown ppt
BiI3 + KI  K[BiI4] soluble
K[BiI4] soluble
BiI3 + H2O  BiOI + 2HI Orange ppt
BiOI + 2HI Orange ppt
COPPER (Cu)
- H2S :
CuCl2 + H2SCuS + 2HCl Black
3CuS + 8NHO3  3Cu(NO3)2 + 2NO + 3S + 4H2O
3Cu(NO3)2 + 2NO + 3S + 4H2O
- NH4OH : 2CuSO4 + NH4OH
Cu(OH)2.CuSO4 + (NH4)2SO4
Blue ppt basic salt
CuSO4.Cu(OH)2 + (NH4)2SO4 + 6NH4OH  2[Cu(NH3)4]SO4+ 8H2O
2[Cu(NH3)4]SO4+ 8H2O
Blue soluble complex
- K4[Fe(CN)6] :
[Cu(NH3)4]SO4 + 4CH3COOHCuSO4 + 4CH3COONH4
2CuSO4 + K4[Fe(CN)6]  Cu2[Fe(CN)6] + 2K2SO4
Cu2[Fe(CN)6] + 2K2SO4
Brown red ppt
- KCN :
[Cu(NH3)4] SO4 + 2KCN + 4 H2O Cu(CN)2 + K2SO4 + 4NH4OH
Cu(CN)2 + K2SO4 + 4NH4OH
2Cu(CN)2  Cu2(CN)2 + (CN)2
Cu2(CN)2 + (CN)2 
Cu(CN)2 + 6KCN  2K3[Cu(CN)4]
2K3[Cu(CN)4]
- Fe :
CuSO4 + Fe  Cu
Cu  + FeSO4
+ FeSO4
The copper complex is very slightly dissociated in soln and the concentration of Cu++ ions is so small that the solubility product of CuS is never reached on passing H2S into the solution
CADMIUM (Cd)
- H2S :
CdCl2 + H2SCdS
+ 2HCl
yellow ppt
3CdS + 8HNO3 3Cd(NO3)2 + 2NO +3S + 4H2O
3Cd(NO3)2 + 2NO +3S + 4H2O
- NH4OH :
CdSO4 + 2NH4OHCd(OH)2
+ (NH4)SO4
White
Cd(OH)2 + (NH4)2SO4 + 2NH4OH  [Cd(NH3)4]SO4 + 4H2O
[Cd(NH3)4]SO4 + 4H2O
complex
- KCN :
[Cd(NH3)4]SO4 + 2KCNCd(CN)2
+ K2SO4 + 4NH3
white
Cd(CN)2 + 2KCN  K2[Cd(CN)4]
K2[Cd(CN)4]
K2[Cd(CN)4 2K+ + [Cd(CN)4]– –
2K+ + [Cd(CN)4]– –
[Cd (CN)4 Cd++ + 4CN–
Cd++ + 4CN–
A sufficient high concentration of Cd++ ions is produced by the dissociation of the complex ion to give ppt of CdS with H2S
K2[Cd(CN)4] +H2S CdS
CdS  + 2KCN + 2HCN
+ 2KCN + 2HCN
DETECTION OF BASIC RADICALS - GROUP IIB
As2S3 yellow
SnS2 yellow
SnS dark brown orange
On treatment with hot (NH4)2S2, the arsenious, antimonious and stannous sulphides are oxidised to higher sulphides and then dissolve to form thio salts
As2S3 + 2(NH4)2S2  2(NH4)2S +As2S5
2(NH4)2S +As2S5
Sb2S3 + 2(NH4)2S2  2(NH4)2S +Sb2S5
2(NH4)2S +Sb2S5
SnS + (NH4)2S2  (NH4)2S +SnS2
(NH4)2S +SnS2
As2S5 + 3(NH4)2S  2(NH4)3AsS4
2(NH4)3AsS4
Amm thio arsenate
Sb2S5 + 3(NH4)2S  2(NH4)3SbS4
2(NH4)3SbS4
Ammthioantimonate
SnS2 + (NH4)2S (NH4)2SnS3
(NH4)2SnS3
Ammthiostannate
Sn2S + (NH4)2S2  (NH4)2SnS3
(NH4)2SnS3
Ammthiostannate
dil HCl decomposes thio salts precipitating sulphides of As, Sb, and Sn. Excess of (NH4)2S is decomposed precipitating sulphur
2(NH4)3 As S4+ 6HCl  AS2S5 + 6NH4Cl + 3H2S
AS2S5 + 6NH4Cl + 3H2S
2(NH4)3SbS4 + 6HCl  Sb2S5 + 6NH4Cl + 3H2S
Sb2S5 + 6NH4Cl + 3H2S
(NH4)2SnS3 + 2HCl  SnS2 + 2NH4Cl + H2S
SnS2 + 2NH4Cl + H2S
(NH4)2S + 2HCl  2NH4Cl + H2S + S
2NH4Cl + H2S + S
Separation : As2S5 is insoluble in conc HCl while SnS2 and Sb2S5 are soluble
Sb2S5 + 6HCl  2SbCl3 + 3H2S + 2S
2SbCl3 + 3H2S + 2S
SbCl3 +HCl  H[SbCl4]
H[SbCl4]
SnCl2 + 2HCl SnCl4 + 2H2S
SnCl4 + 2H2S
SnCl4 + 2HCl  H2[SnCl6]
H2[SnCl6]
To the filtrate iron fillings or granulated zinc is added which reduces SnCl4 to SnCl2
SnCl4 + Zn  SnCl2 + ZnCl2
SnCl2 + ZnCl2
SnCl4 + Fe SnCl2 + FeCl2
SnCl2 + FeCl2
ARSENIC (As)
- Arsenic sulphide dissolves in conc HNO3 to arsenic acid
As2S3 + 10HNO3  2H3AsO4 + 10NO2 + 2H2O + 3S
2H3AsO4 + 10NO2 + 2H2O + 3S
As2S5 + 10HNO3  2H3AsO4 + 10NO2 + 2H2O + 5S
2H3AsO4 + 10NO2 + 2H2O + 5S
Arsenic acid
H3AsO4 + 12(NH4)2MoO4 + 21HNO3  (NH4)3AsO4.12MoO3 + 21NH4NO3 + 12H2O
(NH4)3AsO4.12MoO3 + 21NH4NO3 + 12H2O
Amm. arsenoMolybdate (Yellow ppt)
- As2S3 +3(NH4)2CO3
(NH4)3AsO3 + (NH4)3AsS3 +3CO2
Ammarsenite Ammthioarsenite
As2S5 + 3(NH4)2CO3  (NH4)3AsSO3 + (NH4)3 AsS4 + 3CO2
(NH4)3AsSO3 + (NH4)3 AsS4 + 3CO2
Ammoxythioarsenate Ammthioarsenate
dil HCl decomposes these giving yellow ppt. of As2S3 and As2S5
2(NH4)3 AsS4 + 6HCl As2S5 + 6NH4Cl + 3H2S
As2S5 + 6NH4Cl + 3H2S
2(NH4)3 AsS3 + 6HCl  As2S3 + 6NH4Cl + 3H2S
As2S3 + 6NH4Cl + 3H2S
Amm arsenite and amm. oxy thioarsenate are like wise decomposed
2(NH4)3 AsO3 + 12HCl  2AsCl3 + 6NH4Cl + 6H2O
2AsCl3 + 6NH4Cl + 6H2O
2(NH4)3 AsSO3 + 12HCl 2AsCl3 + 6NH4Cl + 6H2O + S
2AsCl3 + 6NH4Cl + 6H2O + S
When H2S is passed AsCl3 is converted in yellow As2S3
2AsCl3 + 3H2S As2S3 + 6HCl
As2S3 + 6HCl
TIN (Sn)
It forms two types of compounds, stannous and stannic in which it is bivalent and tetravalent.
- H2S :
SnCl2 + H2S  SnS + 2HCl Brown ppt
SnS + 2HCl Brown ppt
SnCl4 + 2H2S  SnS2 + 4HCl Yellow ppt
SnS2 + 4HCl Yellow ppt
SnS does not dissolve in ordinary amm. sulphide and KOH or NaOH while SnS2 dissolves.
SnS +(NH4)2S2  (NH4)2SnS3
(NH4)2SnS3
SnS2 + (NH4)2S  (NH4)2SnS3
(NH4)2SnS3
3SnS2 + 6KOH K2SnO3 + 2K2SnS3 + 3H2O
K2SnO3 + 2K2SnS3 + 3H2O
On acidification SnS2 is precipitated
(NH4)2. SnS3 + 2HCl 2NH4Cl + H2S + SnS2
2NH4Cl + H2S + SnS2
K2SnO3 + 2K2SnS3 + 6HCl 6KCl + 3H2O + 3SnS2
6KCl + 3H2O + 3SnS2
SnS2 + 4HCl  SnCl2 + 2H2S
SnCl2 + 2H2S
SnCl4 + 2HCl  H2[SnCl6]
H2[SnCl6]
- Oxalic acid :
SnCl4 + 4(NH4)2C2O4(NH4)[Sn(C2O4)4(H2O)2] + 4 NH4Cl
Complex ion not decomposed by H2S
- Metals :
SnCl4 + FeSnCl2 + FeCl2
SnCl2 + 2HgCl2  SnCl4 + Hg2Cl2
SnCl4 + Hg2Cl2
White
Hg2Cl2 + SnCl2  SnCl4 + 2Hg
SnCl4 + 2Hg
grey
ANTIMONY (Sb)
- H2S
2 SbCl3 + 3H2SSb2S3 + 6 HCl orange ppt
2 SbCl5 + 5H2S Sb2S5 + 10 HCl
Sb2S5 + 10 HCl 
Sb2S3 + 3(NH4)2S2 2(NH4)3 SbS4 + S Sb2S5 + 3(NH4)2S 2(NH4)3 SbS4
Sb2S5 + 3(NH4)2S 2(NH4)3 SbS4
Sb2S3 + 3(NH4)2S2 2(NH4)3 SbS4 + S
2 Sb2S3 + 4KOH  KSbO2 + 3 KSbS2 + 2 H2O
KSbO2 + 3 KSbS2 + 2 H2O
Sb2S5 + 6KOH K3SbO3 + K3SbS4 + 3 H2O
K3SbO3 + K3SbS4 + 3 H2O
On acidification of the above soln with dil HCl Sb2S5 is precipitated.
2 (NH4)3 SbS4 + 6HC l Sb2S5 + 6 NH4Cl + 3H2S
l Sb2S5 + 6 NH4Cl + 3H2S
K3SbO3 + K3SbS4 + 6HCl  Sb2S5 + 6 KCl + 3H2O
Sb2S5 + 6 KCl + 3H2O
K SbO2 + 3K SbS2 + 4HCl  2Sb2S3 + 4 KCl + 2H2O
2Sb2S3 + 4 KCl + 2H2O
These sulphides dissolve in Conc HCl to form chlorides
Sb2S3 + 6HCl 2SbCl3 + 3H2S
2SbCl3 + 3H2S
Sb2S5 + 6HCl 2SbCl3 + 2S + 3H2S
2SbCl3 + 2S + 3H2S
- Water :
SbCl3 + H2OSbOCl + 2HCl
White ppt antimony oxychloride
- Metals : 2 SbCl3 + 3Fe
Sb + 3FeCl2










