HYBRIDISATION AND SHAPES OF ORGANIC MOLECULES
HYBRIDISATION
Sigma bonds are the most common bonds in organic chemistry. All single bonds are sigma (σ) bonds and formed by the overlapping between s-s, s-p and p-p (head on) atomic orbitals present on different atoms. A pi (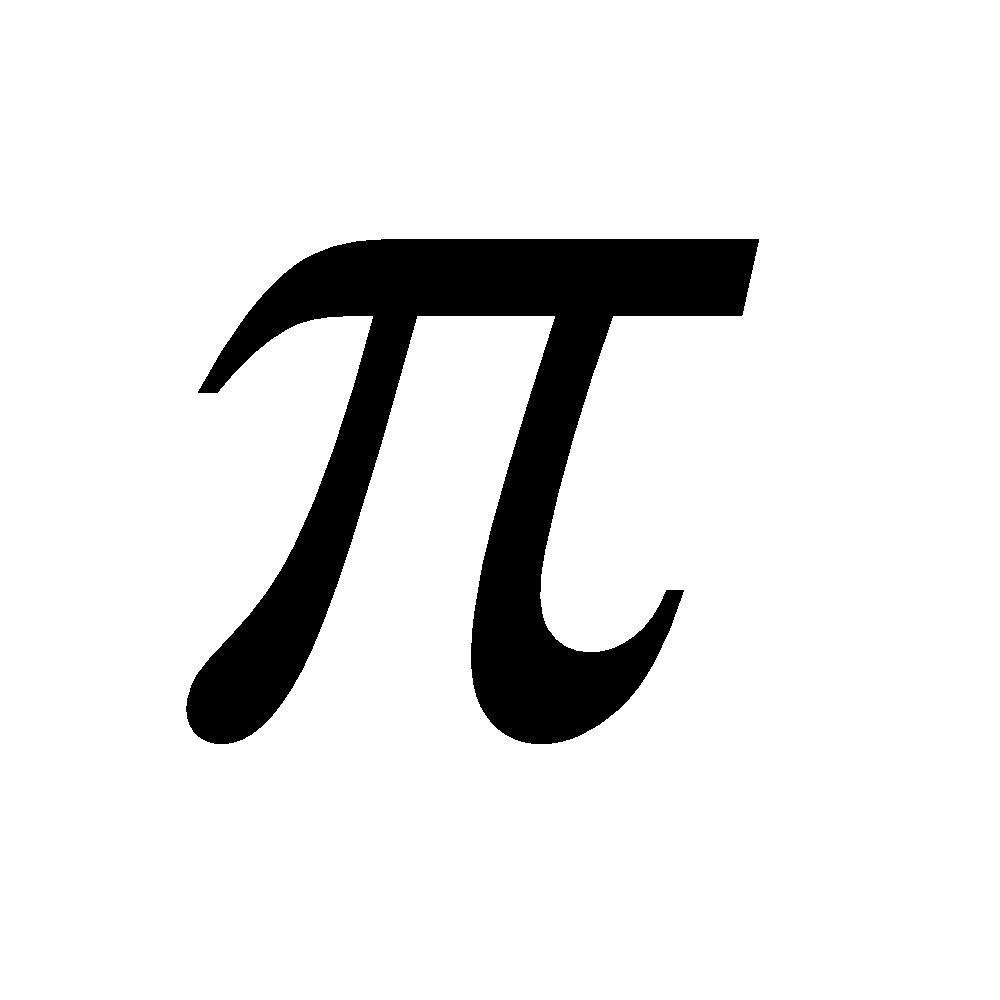 ) bond results from the overlap of two p-orbitals that are oriented perpendicular to the axis of the nuclei. A p bond is not cylindrically symmetrical. A σ bond is stronger than p bond due to better overlap. All multiple bonds contain one σ bond and others
) bond results from the overlap of two p-orbitals that are oriented perpendicular to the axis of the nuclei. A p bond is not cylindrically symmetrical. A σ bond is stronger than p bond due to better overlap. All multiple bonds contain one σ bond and others  bond(s).
bond(s).
To have more efficient overlapping and to provide more symmetrical structure to the molecule the atomic orbitals on the same atom interact to provide hybrid atomic orbitals and the interaction is known as hybridisation. The hybrid atomic orbitals have enhanced electron density.
HYBRIDISATION OF CARBON
The ground state electronic configuration of carbon is  . The electronic configuration of carbon in excited state is
. The electronic configuration of carbon in excited state is  .
.
sp3 HYBRIDISATION
If we superimpose one s and three p atomic orbitals we get 4sp3 hybrid orbitals.
Each hybrid orbital contains single electron, has 25% s character and 75% p character. They are directed towards the four corners of a regular tetrahedron with the carbon located in the centre. The angle between any two sp3 hybrid orbitals is 109º 28' (109.5º).
These hybrid orbitals can overlap with four s atomic orbitals provided by four hydrogen atoms to form methane molecule.
sp2 HYBRIDISATION
If we superimpose one s and two p atomic orbitals we get 3sp2 hybrid orbitals
Each sp2 hybrid orbital has 33% s character and 67% p character. They lie in the same plane with their axis directed towards the corner of an equilateral triangle and are 120º apart from each other. The unhybridized pz atomic orbital is perpendicular to the plane of sp2 hybrid orbitals.
BONDING IN ETHYLENE
Consider two sp2 hybridised carbon atoms approaching to each other and four hydrogen atoms which provide four s atomic orbitals
sp HYBRIDISATION
If we superimpose one s and one p atomic orbitals we get 2sp hybrid orbitals.
Each sp hybrid orbital has 50% s character and 50% p character. They are diagonally present with their axis forming an angle of 180º. The unhybridized 2py and 2pz atomic orbitals are perpendicular to each other and perpendicular to hybrid orbitals also.
BONDING IN ACETYLENE
HYBRIDISATION OF NITROGEN
The ground state electronic configuration of nitrogen is
7N = 1s2, 2s2 2px1 2py1 pz1
One s and three p atomic orbitals superimpose and give 4sp3 hybrid orbitals. These are tetrahedrally present.
sp2 HYBRIDISATION
When nitrogen attaches itself to two other atoms it is present in the sp2 hybridised form. Consider the formation of methylimine CH2 = NH in which carbon and nitrogen both are in sp2 hybrid state
sp HYBRIDISATION
When nitrogen is attached to only one atom its hybridisation is sp. In both carbon and nitrogen are in sp hybridised form
HYBRIDISATION OF OXYGEN
The electronic configuration of oxygen is  .
.
sp3 HYBRIDISATION
When oxygen is attached to two atoms the hybridisation is sp3.
sp2 HYBRIDISATION
When oxygen is attached to one atom as in case of aldehydes and ketones e.g. in Formaldehyde carbon and oxygen, both are in sp2 hybrid form.
BOND LENGTHS
Some importants bond lengths are as follows
C–C sp3 – sp3 1.54 Å C–O sp3 – O 1.41 Å
sp3 – sp2 1.50 Å sp2 – O 1.34 Å
sp3 – sp 1.46 Å C=O sp2 – O 1.20 Å
sp2 – sp2 1.48 Å sp – O 1.16 Å
sp2 – sp 1.43 Å C–N sp3 – N 1.47 Å
sp – sp 1.38 Å sp2 – N 1.36 Å
C=C sp2 – sp2 1.34 Å C=N sp2 – N 1.28 Å
sp2 – sp 1.31 Å CºN sp – N 1.16 Å
sp – sp 1.28 Å
CºC sp – sp 1.21 Å
C–H sp3– H 1.11 Å
sp2 – H 1.10 Å
sp – H 1.08 Å
BOND ANGLES IN SELECTED MOLECULES
AROMATICITY AND AROMATIC COMPOUNDS
Aromatic indicates a stable system which undergoes substitution rather than addition, retaining the closed p-electron system. Many such systems contain only six p electrons, but generally they contain (4n+2) p electrons, where n is an integer.
Non-benzenoid heterocyclic compounds with 6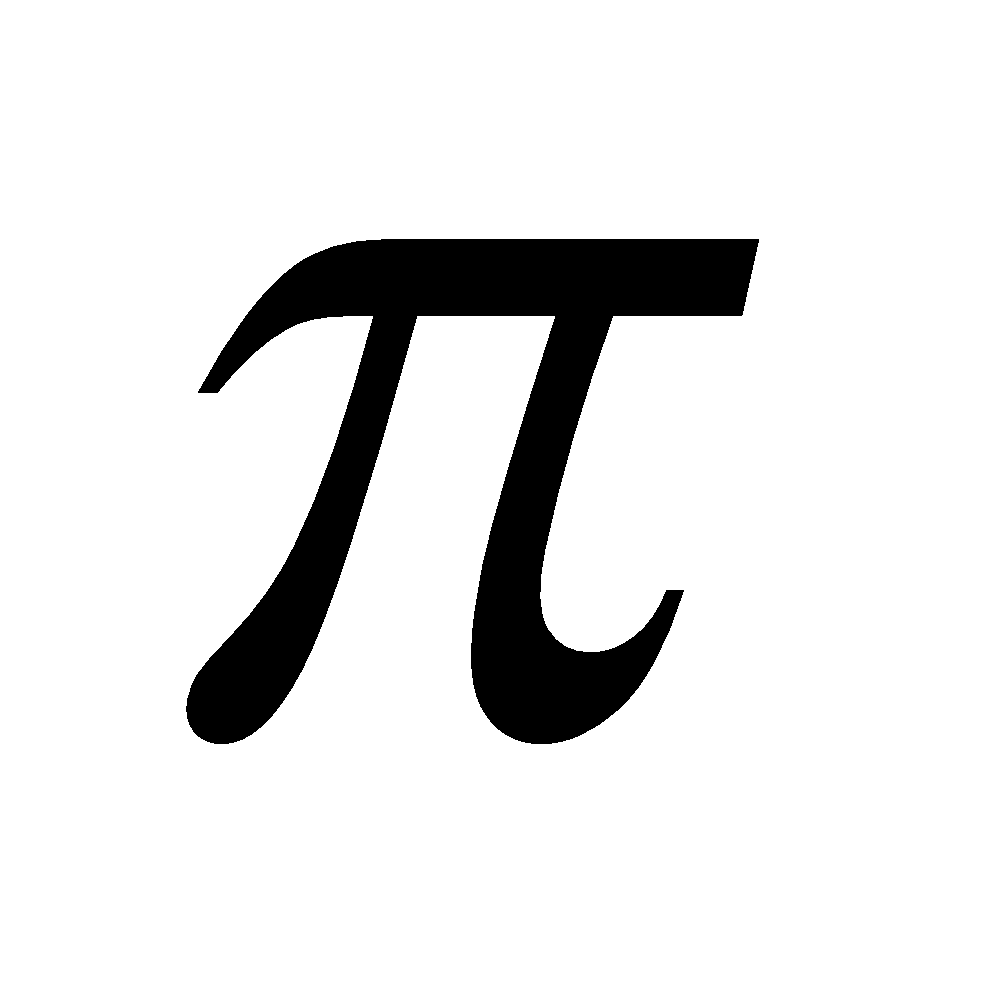 electrons are aromatics
electrons are aromatics
The hetero atom contributes to non bonded electrons, to complete the sextet.
In general, higher polycyclic aromatic compounds are somewhat less stable than benzene.
n = 0 cyclopropenyl cation contains 2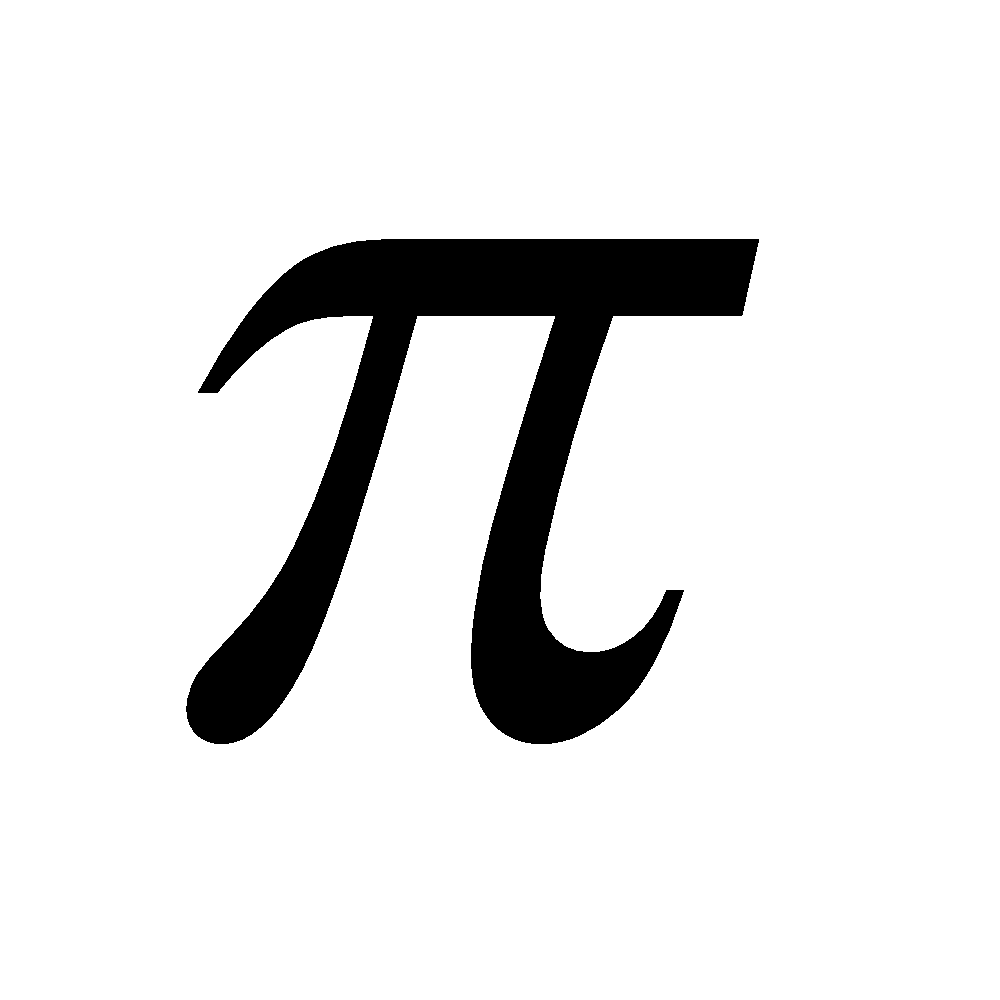 - electrons and is aromatic
- electrons and is aromatic
ANTIAROMATICITY
The less stability of monocyclic compounds containing (4n) 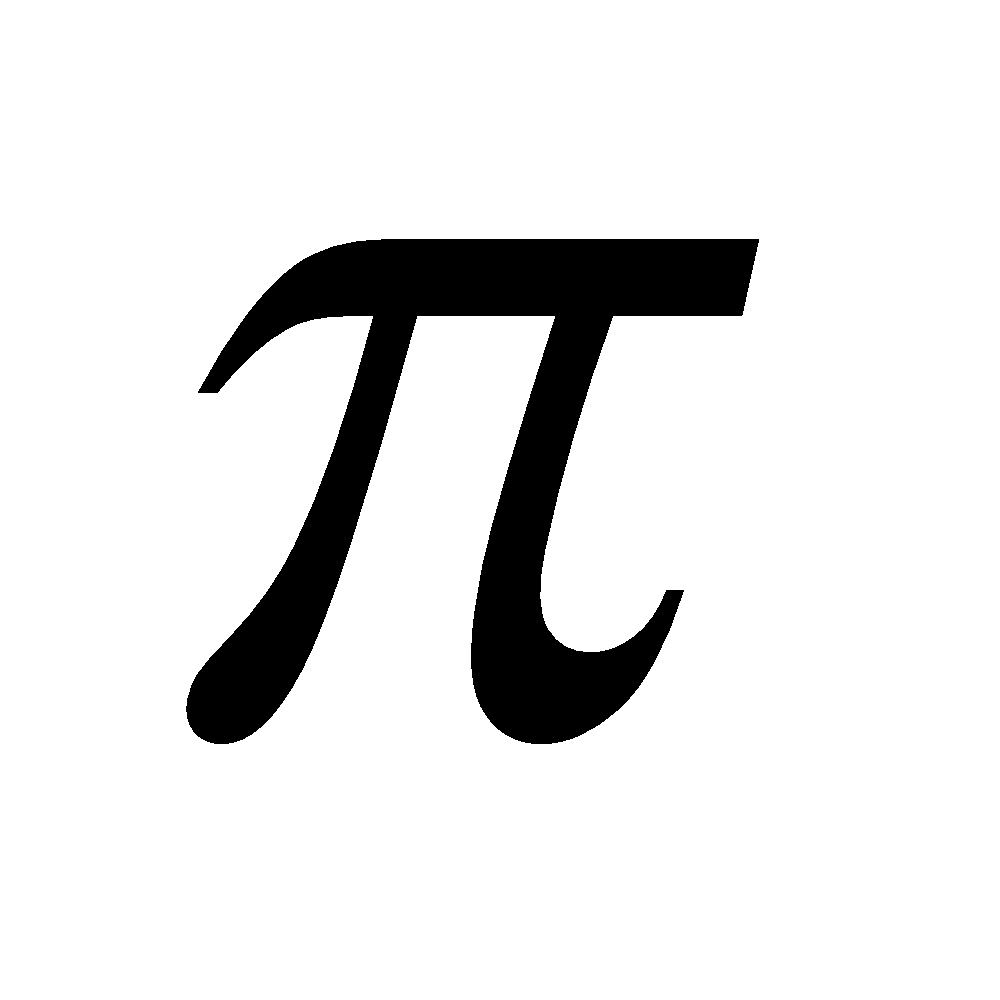 electrons than their acyclic analogues is called anti aromaticity. For example
electrons than their acyclic analogues is called anti aromaticity. For example
Cyclobutadiene  is less stable than 1,3-Butadiene
is less stable than 1,3-Butadiene
Here Resonance is the cause of destabilisation (hence the concept of antiaromaticity)
More examples of antiaromatic compounds
The electronic configuration of carbon in excited state is .










