Important topics for Thermodynamics has been designed in such a way that it offers very practical and application-based learning to further make it easier for students to understand every concept or topic by correlating it with the day-to-day experiences.
Q1. A thermodynamic process is shown in Fig. The pressures and volumes corresponding to some points in the figure are, P_A=3×10^4 Pa,V_A=2×10^(-3) m^3, P_B=8×10^4 Pa,V_D=5×10^(-3) m^3. In process AB, 600 J of heat and in process BC, 200 J of heat is added to the system. The change in the internal energy in process AC would be :
Q2.A vessel contains 1 mole of O_2 gas (molar mass 32) at a temperature T. The pressure of the gas is P. An identical vessel containing one mole of He gas (molar mass 4) at a temperature2 T has a pressure of:
Q3. The internal energy of 3 moles of hydrogen at temperature T is equal to the internal energy of n moles of helium at temperature T/ 2. The value of n is (assume hydrogen and helium to behave like ideal gases):
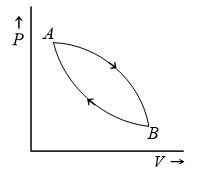
Q4. Figure shows a cyclic process. When a given mass of a gas is expanded from state A to state B, it absorbs 30J of heat energy. When the gas is adiabatically compressed from state B to state A, the work done on the gas is 50 J. The change in internal energy of the e gas in the process A→B is
Q5. During an adiabatic process, the pressure of a gas is proportional to the cube of its absolute temperature. The value of C_p/C_v for that gas is:
Q6. A monoatomic ideal gas, initially at temperature T_1 , is enclosed in a cylinder fitted with a frictionless piston. The gas is allowed to expand adiabatically to a temperature T_2 by releasing the pison suddenly. If L_1 and L_2 are the lengths of the gas column before and after expansion respectively, then T_1/T_2 is given by
Q7. In a given process on an ideal gas, dW=0and dQ<0 .="" for="" gas:="" span="" the="" then="">
Q8. Two cylinders A and B fitted with pistons contain equal amounts of an ideal diatomic gas at 300 K. The piston of A is free to move, while that of B is held fixed. The same amount of heat is given to the gas in each cylinder. If the rise in temperature of the gas in A is 30 K, then the rise in temperature of the gas in B is:
Q9. Two identical containers A and B fitted with frictionless pistons contain the same idal gas at the same temperature and the same volume V. The mass of the gas in A is m_A and that in B is m_B. The gas in each cylinder is now allowed to expand isothermally to the same final volume 2V. The changes in pressure in A and B are found to be ∆P and 1.5 ∆P respectively. Then: