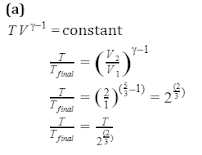IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q1.Which of the following reaction is endothermic?:
Solution
Dissociation of CaCO3 required energy
Dissociation of CaCO3 required energy
Q2.Entropy of system depends upon
Solution
Entropy of system depends upon Pressure, volume, and temperature
Entropy of system depends upon Pressure, volume, and temperature
Q3.When one mole of monoatomic ideal gas at T K undergoes adiabatic change under a constant external pressure of 1 atm changes volume from 1 L to 2 L. The final temperature in Kelvin would be
Q4.H2(g) + 1/2O2(g) → H2O(l)
BE(H-H) = x1; BE(O=O) = x2
BE(O-H) = x3
Latent heat of vaporization of water liquid into water vapour = x4, then ∆fH (heat of formation of liquid water)is
Solution
∆H = BE(reactant) - BE(products)
[But all the species must be in gaseous state. In product,
[H2O(l)⟶H2O(g)] ∆H must be added
Hence, H2(g) + 1/2O2(g)→H2O(l)
∆H=[(BE)H-H + 1/2(BE)O=O]
=[(∆H)vap + 2(BE)O-H]
=x1 + x2/2 - [x4 + 2x3]
=x1 + x2/2 - x4 - 2x3
∆H = BE(reactant) - BE(products)
[But all the species must be in gaseous state. In product,
[H2O(l)⟶H2O(g)] ∆H must be added
Hence, H2(g) + 1/2O2(g)→H2O(l)
∆H=[(BE)H-H + 1/2(BE)O=O]
=[(∆H)vap + 2(BE)O-H]
=x1 + x2/2 - [x4 + 2x3]
=x1 + x2/2 - x4 - 2x3
Q5.Evaporation of water is
Solution
Evaporation of water required heat energy to proceed the reaction
Evaporation of water required heat energy to proceed the reaction
Q6.In thermodynamics, a process is called reversible when
Solution
In thermodynamics, a process is called reversible when the surroundings are always in equilibrium with the system
In thermodynamics, a process is called reversible when the surroundings are always in equilibrium with the system
Q7.Which of the following equations corresponds to the enthalpy of combustion at 298 K?
Solution
Combustion of one mole of reactants gives products in standard state or most stable state
Combustion of one mole of reactants gives products in standard state or most stable state
Q8.If a certain mass of gas is made to undergo separately adiabatic and isothermal expansions to the same pressure, starting from the same initial conditions of temperature and pressure, then, as compared to that of isothermal expansion, in the case of adiabatic expansion, the final
Solution
Volume and temperature will be lower
Volume and temperature will be lower
Q9.Which of the following is an endothermic reaction?
Solution
2H2 + O2 → 2H2O
2H2 + O2 → 2H2O
Q10. The expression ∆sublH⊝ = ∆fusH⊝ + ∆vapH⊝ is true at all
Solution
Temperatures and 1 atm pressure conditions
Temperatures and 1 atm pressure conditions