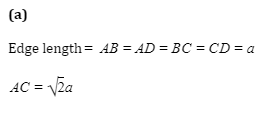IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything...
Q3. The coordination number of a metal crystallizing in a hexagonal close-packed structure is
Solution
(a) In hcp, a particle as shown here is surrounded by 12 particles, six in its own plane and three each above and below the plane
(a) In hcp, a particle as shown here is surrounded by 12 particles, six in its own plane and three each above and below the plane
Q4. The range of radius ratio (cationic to anionic) for an octahedral arrangement of ions in an ionic solid is
Solution
(c) Factual statement
(c) Factual statement
Q5.A semiconductor of Ge can be made p-type by adding
Solution
(a) Ge is Group 14 elements. Positive holes can be created by adding Group 13 element, i.e.,trivalent impurity
(a) Ge is Group 14 elements. Positive holes can be created by adding Group 13 element, i.e.,trivalent impurity
Q6. The material used in solar cells contains
Solution
(b) SiO2 is used in solar cells
(b) SiO2 is used in solar cells
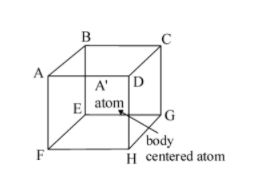
Q7.CsBrhas bcc structure with edge length of 43 pm. The shortest interionic distance between cation and anion is
Q10. How many unit cells are present in a cubic shaped ideal crystal of NaCl of mass 1.0 g?