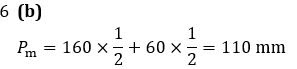IIT JEE exam which consists of JEE Main and JEE Advanced is one of the most important entrance exams for engineering aspirants. The exam is held for candidates who are aspiring to pursue a career in the field of engineering and technical studies.
Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything..
Q2.The osmotic pressure of 40% (weight/volume) urea solution is 1.64 atm and that of 3.42% (weight/volume) cane sugar is 2.46 atm. When equal volumes of the above two solutions are mixed, the osmotic pressure of the resulting solution is
Q4. An ideal solution was obtained by mixing methanol and ethanol. If the partial vapour pressure of methanol and ethanol are 2.619 kPa and 4.556 kPa, respectively, the composition of vapour (in terms of mole fraction) will be
Q5.The colligative properties of a solution depend on
Q6. At 40℃, the vapour pressures of pure liquids, benzene and toluene are 160 mm Hg and 60 mm Hg, respectively. At the same temperature, the vapour pressure of an equimolar solution of the two liquids, assuming the ideal solution, should be
Q7.pH of a 0.1 M monobasic acid is found to be 2. Hence, its osmotic pressure at a given temperature T K is
Q8.Equimolal solutions A and B show depression in freezing point in the ratio 2:1. A remains in the normal state in solution. B will be
Q9.A solution containing 8.6 g urea in 1 L was found to be isotonic with a 5% (weight/volume) solution of organic non-volatile solute. The molecular weight of latter is